Plant Science Research Weekly: July 2, 2021
Review: Functional morphology of plants – a key to biomimetic applications
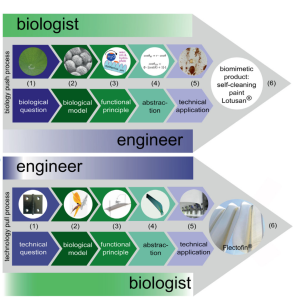 Humans have derived inspiration from innumerable corners of the natural world. Plants of diverse forms have inspired many “biomimetics,” or technical products derived from biological models. Speck and Speck review how plant-based biomimetics have developed over time and elaborate on their current use. An interesting perspective from this review is the generalization of the biomimetic process – the identification of a functional principle, its abstraction into a concept, and finally, its transfer into a technical product. The authors describe these plant-based “functional morphologies” as key to biomimetic developments, citing examples from Velcro (inspired by burdock burrs) to the self-cleaning, water-resistant Lotusan paint (inspired by the wax-repairing function of lotus leaves). Especially pertinent as we face climate change is the use of plant-based biomimetics for sustainable products, although the authors caution “…all sustainability claims can only be fulfilled if a special ethos and a respectful approach to nature complement the technological ambitions in practice.” This paper paints a bright picture of the study plant morphology for the promise of a more sustainable future with plant-based biomimetics. (Summary by Benjamin Jin) New Phytol. 10.1111/nph.17396
Humans have derived inspiration from innumerable corners of the natural world. Plants of diverse forms have inspired many “biomimetics,” or technical products derived from biological models. Speck and Speck review how plant-based biomimetics have developed over time and elaborate on their current use. An interesting perspective from this review is the generalization of the biomimetic process – the identification of a functional principle, its abstraction into a concept, and finally, its transfer into a technical product. The authors describe these plant-based “functional morphologies” as key to biomimetic developments, citing examples from Velcro (inspired by burdock burrs) to the self-cleaning, water-resistant Lotusan paint (inspired by the wax-repairing function of lotus leaves). Especially pertinent as we face climate change is the use of plant-based biomimetics for sustainable products, although the authors caution “…all sustainability claims can only be fulfilled if a special ethos and a respectful approach to nature complement the technological ambitions in practice.” This paper paints a bright picture of the study plant morphology for the promise of a more sustainable future with plant-based biomimetics. (Summary by Benjamin Jin) New Phytol. 10.1111/nph.17396
Real-time conversion of tissue-scale mechanical forces into an interdigitated growth pattern
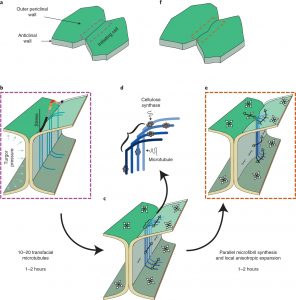 In many plants, epidermal pavement cells lock together through interdigitating lobes, much like the pieces of a jigsaw puzzle. These cells start out as regular squarish cells with straight walls, which then bow out and curve. Here, Belteton et al. investigate how lobes form in cotyledon pavement cells. Using a 3D long-term time-lapse imaging pipeline, they imaged expanding leaves, labeling plasma membranes, microtubules and plasmodesmata; these data allowed them to quantify very localized rates of wall growth in 3D. This pipeline was also used to image cells grown in the presence of various inhibitors as well as cells from mutants associated with wall or cytoskeleton defects. Finite-element modeling of these data simulated stress-strain relationships in the walls. These studies identified a critical role in lobe formation of microtubules that are transfacial: i.e., they span both periclinal (parallel to the leaf surface) and anticlinal (perpendicular to the leaf surface) surfaces. These localized microtubule arrays pattern cellulose microfibrils, causing localized asymmetry which leads to lobe formation. These transfacial microtubles can coordinate the growth rate of upper and lower periclinal walls, allowing the anticlinal wall to maintain a constant tilt angle. This mechanistic model of lobe formation provides a foundation to analyse the cellular basis of leaf morphology and function. (Summary by Mary Williams @PlantTeaching) Nature Plants 10.1038/s41477-021-00931-z
In many plants, epidermal pavement cells lock together through interdigitating lobes, much like the pieces of a jigsaw puzzle. These cells start out as regular squarish cells with straight walls, which then bow out and curve. Here, Belteton et al. investigate how lobes form in cotyledon pavement cells. Using a 3D long-term time-lapse imaging pipeline, they imaged expanding leaves, labeling plasma membranes, microtubules and plasmodesmata; these data allowed them to quantify very localized rates of wall growth in 3D. This pipeline was also used to image cells grown in the presence of various inhibitors as well as cells from mutants associated with wall or cytoskeleton defects. Finite-element modeling of these data simulated stress-strain relationships in the walls. These studies identified a critical role in lobe formation of microtubules that are transfacial: i.e., they span both periclinal (parallel to the leaf surface) and anticlinal (perpendicular to the leaf surface) surfaces. These localized microtubule arrays pattern cellulose microfibrils, causing localized asymmetry which leads to lobe formation. These transfacial microtubles can coordinate the growth rate of upper and lower periclinal walls, allowing the anticlinal wall to maintain a constant tilt angle. This mechanistic model of lobe formation provides a foundation to analyse the cellular basis of leaf morphology and function. (Summary by Mary Williams @PlantTeaching) Nature Plants 10.1038/s41477-021-00931-z
Innovation, conservation, and repurposing of gene function in root cell type development
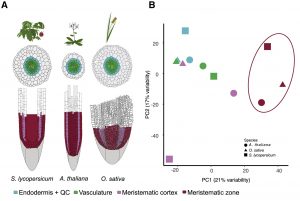 Plants demonstrate a gorgeous diversity in cell types to adapt to their unique environments. Certain cell types, such as the epidermal, cortex, and vascular cells within plant roots, or the classically-defined root developmental zones (meristematic, elongation, and maturation), are homologous in angiosperms. However, how tightly they are conserved across species is unknown. To give insight into the molecular conservation of diverse root cell types across plants, Kajala et al. performed a wide-scale study using translating ribosome affinity purification (TRAP) on a variety of cell types in different developmental stages from tomato, rice, and Arabidopsis. Using the TRAP method, cell type-enriched groups (CTEGs) that display significant translatome enrichment between cell types were analyzed. Through the power of this technique across multiple plant species under different conditions, the authors made many interesting discoveries. One is the “repurposing” of genes: genes or networks that have been adapted for different functions, such as nitrogen regulation within the tomato exodermis, a root tissue not present in Arabidopsis. The authors also observed conservation or partial conservation of xylem gene regulators. A final noteworthy conclusion is the variation in translatomes between sterile and soil growth conditions of Arabidopsis, and between Arabidopsis root translatomes and those of other species. (Summary by Benjamin Jin) Cell 10.1016/j.cell.2021.04.024
Plants demonstrate a gorgeous diversity in cell types to adapt to their unique environments. Certain cell types, such as the epidermal, cortex, and vascular cells within plant roots, or the classically-defined root developmental zones (meristematic, elongation, and maturation), are homologous in angiosperms. However, how tightly they are conserved across species is unknown. To give insight into the molecular conservation of diverse root cell types across plants, Kajala et al. performed a wide-scale study using translating ribosome affinity purification (TRAP) on a variety of cell types in different developmental stages from tomato, rice, and Arabidopsis. Using the TRAP method, cell type-enriched groups (CTEGs) that display significant translatome enrichment between cell types were analyzed. Through the power of this technique across multiple plant species under different conditions, the authors made many interesting discoveries. One is the “repurposing” of genes: genes or networks that have been adapted for different functions, such as nitrogen regulation within the tomato exodermis, a root tissue not present in Arabidopsis. The authors also observed conservation or partial conservation of xylem gene regulators. A final noteworthy conclusion is the variation in translatomes between sterile and soil growth conditions of Arabidopsis, and between Arabidopsis root translatomes and those of other species. (Summary by Benjamin Jin) Cell 10.1016/j.cell.2021.04.024
Deceleration of cell cycle underpins a switch from proliferative-to-terminal division in plant stomatal lineage
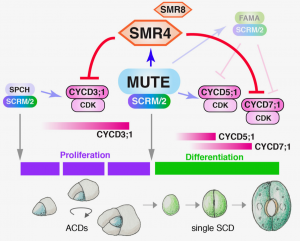 Precise cell cycle control is essential for the differentiation of specialized cell types. Stomata, essential pores for gas exchange located in the epidermis, are generated via proliferative asymmetric cell division (ACD) of a transient stem cell called a meristemoid, which will give rise to a guard mother cell that will undergo a single symmetric cell division (SCD) to generate a pair of guard cells that will terminally differentiate into a mature stoma. However, although the general developmental pathway for stomata development is well understood, how the precise mechanism that controls the switch from proliferative ACDs-to-SCD is currently obscure. A recent paper by Han and colleagues sheds light on the matter by identifying the cyclin-dependent kinase inhibitor (CKI) SIAMESE-RELATED4 (SMR4) as a key player for the ACD-to-SCD transition during stomata development. After having observed through time-lapse imaging that cell cycle duration during ACD is shorter than during SCD, the authors turned their attention to CKIs, well-known negative regulators of cell cycle progression. Transcriptome and ChIP-seq analyses identified the SMR4 CKI as a direct target of MUTE, master transcription factor key for the ACD-to-SCD switch. Subsequent analyses using fluorescent reporter lines revealed that SMR4 is expressed during late meristemoid development through SCD, while loss-of-function (LOF) smr4 mutants showed an increase in the number of stomata and stomata-precursor cells, indicating that SMR4 acts as a repressor of ACD. Furthermore, the authors measured cell cycle duration in plants ectopically expressing SMR4 and discovered that indeed SMR4 significantly slowed down the cell cycle through G1 extension. Finally, the authors showed that SMR4 exerts its function by physically interacting with the essential ACD and SCD regulators CYCD3;1 and CYCD7;1, respectively, as evidenced by the fact that ectopic expression of CYCD3;1 and CYCD7;1 mimics the LOF smr4 phenotype (increased number of stomata and stomata-precursor cells). This elegant work adds to the nascent notion that particular combinations of cell cycle regulators deployed at highly specific developmental stages carry out crucial roles in maintaining the proliferation/differentiation balance. (Summary by Jesus Leon @jesussaur) bioRxiv 10.1101/2021.05.17.442671
Precise cell cycle control is essential for the differentiation of specialized cell types. Stomata, essential pores for gas exchange located in the epidermis, are generated via proliferative asymmetric cell division (ACD) of a transient stem cell called a meristemoid, which will give rise to a guard mother cell that will undergo a single symmetric cell division (SCD) to generate a pair of guard cells that will terminally differentiate into a mature stoma. However, although the general developmental pathway for stomata development is well understood, how the precise mechanism that controls the switch from proliferative ACDs-to-SCD is currently obscure. A recent paper by Han and colleagues sheds light on the matter by identifying the cyclin-dependent kinase inhibitor (CKI) SIAMESE-RELATED4 (SMR4) as a key player for the ACD-to-SCD transition during stomata development. After having observed through time-lapse imaging that cell cycle duration during ACD is shorter than during SCD, the authors turned their attention to CKIs, well-known negative regulators of cell cycle progression. Transcriptome and ChIP-seq analyses identified the SMR4 CKI as a direct target of MUTE, master transcription factor key for the ACD-to-SCD switch. Subsequent analyses using fluorescent reporter lines revealed that SMR4 is expressed during late meristemoid development through SCD, while loss-of-function (LOF) smr4 mutants showed an increase in the number of stomata and stomata-precursor cells, indicating that SMR4 acts as a repressor of ACD. Furthermore, the authors measured cell cycle duration in plants ectopically expressing SMR4 and discovered that indeed SMR4 significantly slowed down the cell cycle through G1 extension. Finally, the authors showed that SMR4 exerts its function by physically interacting with the essential ACD and SCD regulators CYCD3;1 and CYCD7;1, respectively, as evidenced by the fact that ectopic expression of CYCD3;1 and CYCD7;1 mimics the LOF smr4 phenotype (increased number of stomata and stomata-precursor cells). This elegant work adds to the nascent notion that particular combinations of cell cycle regulators deployed at highly specific developmental stages carry out crucial roles in maintaining the proliferation/differentiation balance. (Summary by Jesus Leon @jesussaur) bioRxiv 10.1101/2021.05.17.442671
Building back more equitable STEM education: Teach science by engaging students in doing science
 I firmly believe that the experience of doing science (observing, framing questions, designing and carrying out experiments, and interpreting findings) is the best way to cultivate future scientists, as well as a science-literate public (which we so clearly need). This preprint by Elgin et al. (it’s not often we have a first author with a Wikipedia page, check it out), makes a case for a greatly increased adoption of CURES – Course-based Undergraduate Research Experiences. What are CUREs? Imagine your typical undergraduate lab class, but instead of following a lab manual to a fixed point, the students get to actively participate in a guided research experience – in other words, they get to be a scientist. Fortunately, there is now a substantial database of CUREs that you can draw on and roll out for your students. To ensure access to these valuable experiences, the authors propose the formation of a National Center for Science Engagement, to stimulate and assist in the development and support of CUREs. These rich experiences should be available to all, even those at resource-limited institutions, so broadening their implementation will help to dismantle the inequities that limit full participation in science. (Note that many conferences including ICAR2021 and PlantBio21 are hosting workshops about plant science CUREs). This is an exciting proposal and all I can say is “Bravo!” (Summary by Mary Williams @PlantTeaching) bioRxiv 10.1101/2021.06.01.446616
I firmly believe that the experience of doing science (observing, framing questions, designing and carrying out experiments, and interpreting findings) is the best way to cultivate future scientists, as well as a science-literate public (which we so clearly need). This preprint by Elgin et al. (it’s not often we have a first author with a Wikipedia page, check it out), makes a case for a greatly increased adoption of CURES – Course-based Undergraduate Research Experiences. What are CUREs? Imagine your typical undergraduate lab class, but instead of following a lab manual to a fixed point, the students get to actively participate in a guided research experience – in other words, they get to be a scientist. Fortunately, there is now a substantial database of CUREs that you can draw on and roll out for your students. To ensure access to these valuable experiences, the authors propose the formation of a National Center for Science Engagement, to stimulate and assist in the development and support of CUREs. These rich experiences should be available to all, even those at resource-limited institutions, so broadening their implementation will help to dismantle the inequities that limit full participation in science. (Note that many conferences including ICAR2021 and PlantBio21 are hosting workshops about plant science CUREs). This is an exciting proposal and all I can say is “Bravo!” (Summary by Mary Williams @PlantTeaching) bioRxiv 10.1101/2021.06.01.446616
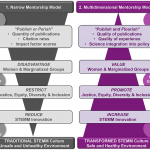
Promoting inclusive metrics of success and impact to dismantle a discriminatory reward system in science
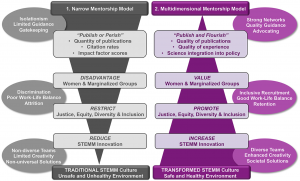 As scientists, we often measure what we can measure as a proxy for what we want to measure. Critical to this strategy is a good understanding of how well the first represents the second. Currently, advancement in science largely rests upon factors that are easy to measure: number of publications and their citations. Here, Davies, Putnam, et al. highlight the fact that these metrics are “flawed and biased against already marginalized groups and fail to accurately capture the breadth of individuals’ meaningful scientific impacts.” This narrow vision of success disadvantages women and marginalized scientists, restricts justice, equity, diversity and inclusion, and reduces innovation. A scientist’s “value” extends far beyond these citation numbers and include dissemination, collaboration, communication, and training, all of which should be embraced and rewarded. The authors provide suggestions for how to shift to a “publish and flourish” model, highlighting the training and support of inclusive mentoring that includes sponsoring, counseling, networking, and advocating. These changes will require many cultural shifts that must be embraced by all and led by those currently in positions of power and privilege. (Summary by Mary Williams @PlantTeaching) PLOS Bio 10.1371/journal.pbio.3001282
As scientists, we often measure what we can measure as a proxy for what we want to measure. Critical to this strategy is a good understanding of how well the first represents the second. Currently, advancement in science largely rests upon factors that are easy to measure: number of publications and their citations. Here, Davies, Putnam, et al. highlight the fact that these metrics are “flawed and biased against already marginalized groups and fail to accurately capture the breadth of individuals’ meaningful scientific impacts.” This narrow vision of success disadvantages women and marginalized scientists, restricts justice, equity, diversity and inclusion, and reduces innovation. A scientist’s “value” extends far beyond these citation numbers and include dissemination, collaboration, communication, and training, all of which should be embraced and rewarded. The authors provide suggestions for how to shift to a “publish and flourish” model, highlighting the training and support of inclusive mentoring that includes sponsoring, counseling, networking, and advocating. These changes will require many cultural shifts that must be embraced by all and led by those currently in positions of power and privilege. (Summary by Mary Williams @PlantTeaching) PLOS Bio 10.1371/journal.pbio.3001282