Plant PIEZO homologs modulate vacuole morphology during tip growth (Science)
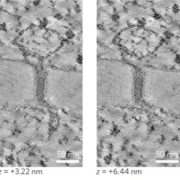
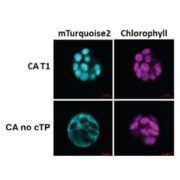
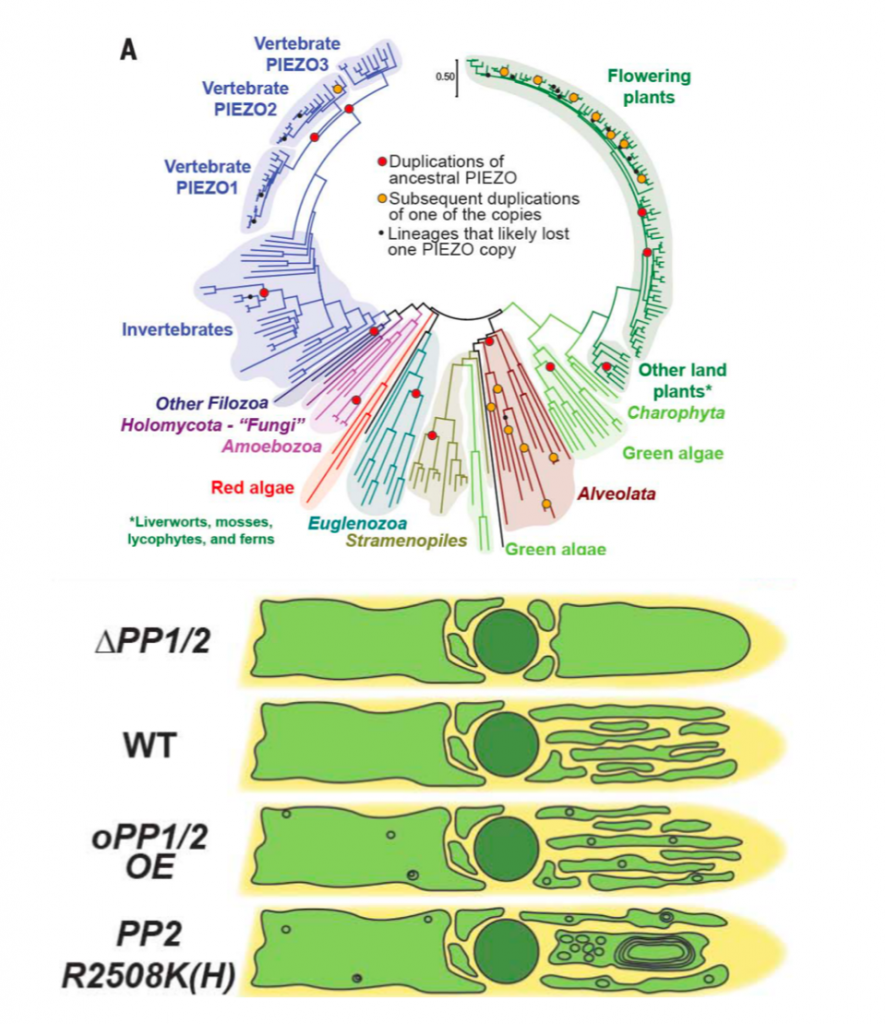 The ability to perceive and respond to mechanical stimuli and forces, such as gravity or touch, is an extremely well conserved property that is key for proper cellular function. This ability is fueled by mechanosensitive ion channels activated by mechanical disturbances in the membrane, which leads to flow of ions down their electrochemical gradients. In animals, the PIEZO family of mechanosensitive channels located in the plasma membrane conduct calcium and are necessary for light touch perception, compressive force, proprioception, nociception, among other processes. Interestingly, although PIEZO homologs can be found in genomes across the eukaryotic tree of life, the biomechanics of the plant cell imposed by its cell wall pose an interesting question: how might PIEZO channels function in plants? Seeking to answer this question, Radin and colleagues recently investigated the PIEZO homologs of the moss Physcomitrium patens (PpPIEZOs) and Arabidopsis thaliana (AtPIEZO) using different types of mutants (loss-of-function [LOF], gain-of-function, overexpression), reporter lines and vacuolar structure-specific dyes. The authors discovered that PpPIEZO proteins localize to the vacuole membrane (tonoplast) in subapical and apical caulonemal cells and are required for proper growth and cytoplasmic Ca2+ oscillation signatures. In the wild-type, caulonemal cells contain tubulated and fragmented vacuoles, in contrast, the vacuoles in the LOF mutant background are fused and expanded, therefore suggesting that PpPIEZOs are necessary for normal tubulated vacuoles. The authors were able to recapitulate the LOF phenotype using a gain-of-function mutant in which a key amino acid residue was deleted, thus further supporting their conclusions: PpPIEZOs influence vacuolar fission/morphology. Finally, the authors assessed the conservation of the mechanism of PIEZO proteins mechanism by AtPIEZO in moss: indeed, AtPIEZO localizes to the tonoplast and suppresses the vacuolar defects observed in the PpPIEZO LOF background. Moreover, AtPIEZO LOF mutants show similar vacuolar defects as the ones observed in moss, therefore suggesting that the role of PIEZO proteins in promoting vacuolar fission and invagination is conserved between moss and Arabidopsis. This interesting work spotlights how PIEZO plant homologs may have been co-opted to sense the mechanical of the tonoplast, rather than of the plasma membrane like in animals. (Summary by Jesus Leon @jesussaur) Science 10.1126/science.abe6310
The ability to perceive and respond to mechanical stimuli and forces, such as gravity or touch, is an extremely well conserved property that is key for proper cellular function. This ability is fueled by mechanosensitive ion channels activated by mechanical disturbances in the membrane, which leads to flow of ions down their electrochemical gradients. In animals, the PIEZO family of mechanosensitive channels located in the plasma membrane conduct calcium and are necessary for light touch perception, compressive force, proprioception, nociception, among other processes. Interestingly, although PIEZO homologs can be found in genomes across the eukaryotic tree of life, the biomechanics of the plant cell imposed by its cell wall pose an interesting question: how might PIEZO channels function in plants? Seeking to answer this question, Radin and colleagues recently investigated the PIEZO homologs of the moss Physcomitrium patens (PpPIEZOs) and Arabidopsis thaliana (AtPIEZO) using different types of mutants (loss-of-function [LOF], gain-of-function, overexpression), reporter lines and vacuolar structure-specific dyes. The authors discovered that PpPIEZO proteins localize to the vacuole membrane (tonoplast) in subapical and apical caulonemal cells and are required for proper growth and cytoplasmic Ca2+ oscillation signatures. In the wild-type, caulonemal cells contain tubulated and fragmented vacuoles, in contrast, the vacuoles in the LOF mutant background are fused and expanded, therefore suggesting that PpPIEZOs are necessary for normal tubulated vacuoles. The authors were able to recapitulate the LOF phenotype using a gain-of-function mutant in which a key amino acid residue was deleted, thus further supporting their conclusions: PpPIEZOs influence vacuolar fission/morphology. Finally, the authors assessed the conservation of the mechanism of PIEZO proteins mechanism by AtPIEZO in moss: indeed, AtPIEZO localizes to the tonoplast and suppresses the vacuolar defects observed in the PpPIEZO LOF background. Moreover, AtPIEZO LOF mutants show similar vacuolar defects as the ones observed in moss, therefore suggesting that the role of PIEZO proteins in promoting vacuolar fission and invagination is conserved between moss and Arabidopsis. This interesting work spotlights how PIEZO plant homologs may have been co-opted to sense the mechanical of the tonoplast, rather than of the plasma membrane like in animals. (Summary by Jesus Leon @jesussaur) Science 10.1126/science.abe6310