A Histone Chaperone and a Specific Transcription Factor Modulate GLABRA2 Expression in Root Hair Development
IN BRIEF by Jennifer Mach [email protected]
 To navigate its essential function of producing mRNAs, RNA polymerase II (Pol II) must navigate the thread of DNA, which winds around thousands of nucleosomes. If you’ve ever tried to use a sewing machine but got your bobbin thread tangled, then the task faced by Pol II may seem impossible. However, Pol II has help: Histone chaperones and chromatin-remodeling complexes remove nucleosomes from its path; they also slide nucleosomes, prevent the aggregation of free histones with DNA, and exchange nucleosomes with histone variants (reviewed in Venkatesh and Workman, 2015). Some chaperones interact preferentially with different histones; for example, H2A/H2B chaperone interact with the H2A/H2B dimers that flank the (H3-H4)2 tetramer in the histone core.
To navigate its essential function of producing mRNAs, RNA polymerase II (Pol II) must navigate the thread of DNA, which winds around thousands of nucleosomes. If you’ve ever tried to use a sewing machine but got your bobbin thread tangled, then the task faced by Pol II may seem impossible. However, Pol II has help: Histone chaperones and chromatin-remodeling complexes remove nucleosomes from its path; they also slide nucleosomes, prevent the aggregation of free histones with DNA, and exchange nucleosomes with histone variants (reviewed in Venkatesh and Workman, 2015). Some chaperones interact preferentially with different histones; for example, H2A/H2B chaperone interact with the H2A/H2B dimers that flank the (H3-H4)2 tetramer in the histone core.
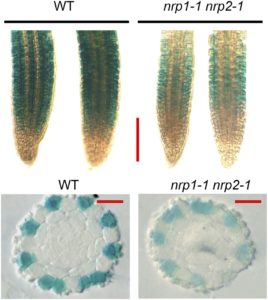
Histone chaperones affect transcription, DNA replication, and DNA repair and play important roles in plant growth and development (reviewed in Zhou et al., 2015). For example, mutants of the histone H2A/H2B chaperone genes NUCLEOSOME ASSEMBLY PROTEIN1-RELATED PROTEIN1 (NRP1) and NRP2 produce ectopic root hairs. Root hairs develop from single epidermal cells (H-cells). In Arabidopsis thaliana, H-cells develops over the junctions between pairs of cortical cells; epidermal cells not directly over the junction (N-cells) do not generally form root hairs. The homeodomain-leucine zipper transcription factor GLABRA2 (GL2) suppresses formation of root hairs in N-cells, and root hair specification involves changes in GL2 transcription. Zhu et al. (2017) examined the role of NRP1 and NRP2 in root hair development and found that the nrp1-1 nrp2-1 mutants have lower GL2 expression, although GL expression occurs in the same cells.
The basic helix-loop-helix transcription factor WEREWOLF (WER) forms part of a complex that activates GL2 expression in N-cells, and the transcript levels of WER and other upstream regulators of GL2 remained unchanged in the nrp1-1 nrp2-1 mutants. Also, the wer-1 nrp1-1 nrp2-1 triple mutants had more root hairs than the nrp1-1 nrp2-1 double mutants, similar to the wer-1 single mutants, suggesting that wer-1 is epistatic. The authors next used chromatin immunoprecipitation-PCR to show that association of NRP1 with the GL2 promoter decreased significantly in the wer-1 mutants. Pull-down and bimolecular fluorescence complementation assays showed that WER and NRP1 directly interact; size-exclusion chromatography indicated that WER and NRP1 form a 1:1 complex and that NRP1 forms dimers. Indeed, determination of the crystal structure of NRP1 showed that NRP1 forms dimers through an N-terminal α-helix. Mutations that disrupt the ability of NRP1 to form dimers and mutants that disrupt its acidic C terminus decrease NPR1’s interaction with histones and with WER1. Moreover, these mutants do not rescue the nrp1-1 nrp2-1 phenotype.
These results linked the NRP1 and NRP2 histone chaperones with WER; the authors next explored how these factors affect chromatin structure at GL2. They found that the GL2 promoter had higher histone occupancy and nucleosome density (and was less accessible to micrococcal nuclease) in the nrp1-1 nrp2-1 mutants than in the wild type. Other loci, such as ACTIN2 and FLOWERING LOCUS C, did not show a difference between the mutant and wild type. Finally, electrophoretic mobility shift assays showed that the presence of histones decreased binding of WER to its target, but NRP1 removed the histones and allowed WER to bind.
This study used a wide-range of techniques, including genetics, molecular biology, and crystallography, to examine how the interaction of histone chaperones with the WER transcription factor targets these chaperones to GL2 and alters the chromatin there. Determining how generally this mechanism occurs, what other chromatin factors affect GL2, and how NRPs regulate other loci will provide intriguing topics for future work.
Venkatesh, S., Workman, J.L. (2015). Histone exchange, chromatin structure, and the regulation of transcription. Nat. Rev. Mol. Cell Biol. 16: 178–189.
Zhou, W., Zhu, Y., Dong, A., Shen, W.H. (2015). Histone H2A/H2B chaperones: from molecules to chromatin-based functions in plant growth and development. Plant J. 83: 78–95.
Zhu, Y., Rong, L., Luo, Q., Wang, B., Zhou, N., Yang, Y., Zhang, C., Feng, H., Zheng, L., Shen, W.-H., Ma, J., Dong, A. (2017). The histone chaperone NRP1 interacts with WEREWOLF to activate GLABRA2 in Arabidopsis root hair development. Plant Cell 29: 260–276.


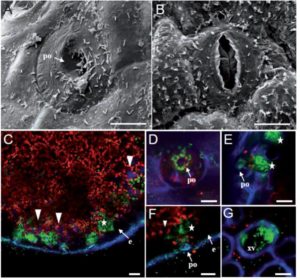 Hydathodes are the sites of guttation, which is a process by which water and solutes are pushed out of leaves by the force of root pressure when the rate of transpiration is low (for example at night). Hydathodes have numerous stomata-like pores and are located near vascular ends. Like stomata, hydathodes provide infection opportunities for pathogens. Cerutti et al. examine the physiology and anatomy of hydathodes in Arabidopsis and cauliflower (Brassica oleracea var. botrytis). They show that like stomata, hydathodes respond to light, CO2 and ABA. Upon inoculation with pathogens or the epitope flg22, there was no evidence for pre-invasive immunity at hydathodes, although post-invasive immunity including production of reactive oxygen species and development of necrotic lesions was observed. Plant Physiol.
Hydathodes are the sites of guttation, which is a process by which water and solutes are pushed out of leaves by the force of root pressure when the rate of transpiration is low (for example at night). Hydathodes have numerous stomata-like pores and are located near vascular ends. Like stomata, hydathodes provide infection opportunities for pathogens. Cerutti et al. examine the physiology and anatomy of hydathodes in Arabidopsis and cauliflower (Brassica oleracea var. botrytis). They show that like stomata, hydathodes respond to light, CO2 and ABA. Upon inoculation with pathogens or the epitope flg22, there was no evidence for pre-invasive immunity at hydathodes, although post-invasive immunity including production of reactive oxygen species and development of necrotic lesions was observed. Plant Physiol. 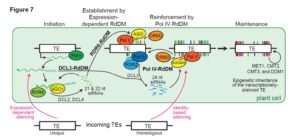 Fultz and Slotkin explore the question of how transposable element (TE) silencing is initiated. As they describe, there are two recognized mechanisms, one based on TE identity (meaning that it can be silenced through siRNAs initiated from a related TE, and in which de novo silencing can occur in the absence of mRNA production), and one based on expression level. To separate these pathways, the authors introduced exogenous TEs that have no sequence identity to TEs in the cell; this strategy therefore excludes the identity-based silencing pathway. Key findings include the different fates of full-length TEs as compared to TE fragments, and different outcomes when TEs are introduced by introgression (crossing) or direct transformation. Plant Cell
Fultz and Slotkin explore the question of how transposable element (TE) silencing is initiated. As they describe, there are two recognized mechanisms, one based on TE identity (meaning that it can be silenced through siRNAs initiated from a related TE, and in which de novo silencing can occur in the absence of mRNA production), and one based on expression level. To separate these pathways, the authors introduced exogenous TEs that have no sequence identity to TEs in the cell; this strategy therefore excludes the identity-based silencing pathway. Key findings include the different fates of full-length TEs as compared to TE fragments, and different outcomes when TEs are introduced by introgression (crossing) or direct transformation. Plant Cell  Familiar citrus fruits such as sweet orange, lemon, lime and grapefruit are hybrids of three species: Citrus reticulate (mandarin), C. medica (citron), and C. maxima (pummelo). Cultivated varieties are generally vegetatively propagated, with diversity arising from spontaneous or induced somatic mutations. Butelli et al. previously showed that anthocyanin accumulation in sweet orange is correlated with expression of the Ruby gene, which encodes a MYB transcription factor. In this study, they extend their characterization of Ruby across Citrus species and hybrids. Their study indicates that the as a result of mutations in the Ruby gene, anthocyanin production s has been lost independently several times in Citrus and its close relatives. Phylogenetic analysis of the Ruby gene also helps to clarify the relationships between citrus varieties. Plant Physiol.
Familiar citrus fruits such as sweet orange, lemon, lime and grapefruit are hybrids of three species: Citrus reticulate (mandarin), C. medica (citron), and C. maxima (pummelo). Cultivated varieties are generally vegetatively propagated, with diversity arising from spontaneous or induced somatic mutations. Butelli et al. previously showed that anthocyanin accumulation in sweet orange is correlated with expression of the Ruby gene, which encodes a MYB transcription factor. In this study, they extend their characterization of Ruby across Citrus species and hybrids. Their study indicates that the as a result of mutations in the Ruby gene, anthocyanin production s has been lost independently several times in Citrus and its close relatives. Phylogenetic analysis of the Ruby gene also helps to clarify the relationships between citrus varieties. Plant Physiol. 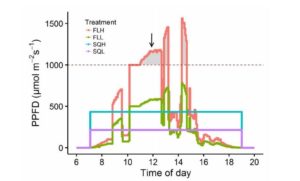 Plant growth chambers are indispensable for most plant science researchers, but of course they do not replicate the outdoor environment. Vialet-Chabrand and Matthews et al. explore the effect of realistic, dynamic fluctuating light (for example as influenced by clouds and leaves) versus light provided in more static conditions (square wave). Plants grown under fluctuating light produced significantly smaller leaves and showed a 20% reduction in biomass accumulation as compared to those grown under square-wave light. However, the fluctuating light grown phenotype enabled these plants to perform more effectively in dynamic environments. These studies illustrate that the typical growth-chamber light regime has limitations in studies that aim to infer plant productivity in natural conditions. Plant Physiol.
Plant growth chambers are indispensable for most plant science researchers, but of course they do not replicate the outdoor environment. Vialet-Chabrand and Matthews et al. explore the effect of realistic, dynamic fluctuating light (for example as influenced by clouds and leaves) versus light provided in more static conditions (square wave). Plants grown under fluctuating light produced significantly smaller leaves and showed a 20% reduction in biomass accumulation as compared to those grown under square-wave light. However, the fluctuating light grown phenotype enabled these plants to perform more effectively in dynamic environments. These studies illustrate that the typical growth-chamber light regime has limitations in studies that aim to infer plant productivity in natural conditions. Plant Physiol. 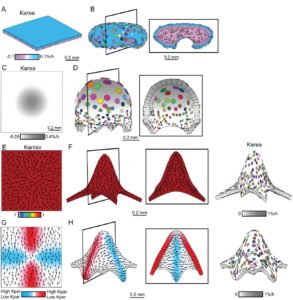 It’s easy to visualize how a sheet of cells grows, but how does a sheet of cells form a complex, three-dimensional structure? Rebocho et al. describe how differential growth rates between cell layers and across the growing surface can produce a variety of complex shapes. As a model for shape complexity, the authors examine growth patterns in snapdragon (Antirrhinum majus) flowers (wild-type and mutant), and through their analysis along with computational modeling show that “orthogonal directional conflict plays a key role in generating out-of-plane deformations”. They further propose how gene activity, mechanical connectivity and the auxin transporter PIN1 can contribute to the generation of tissue conflicts and therefore shape. Don’t miss the movies! eLIFE
It’s easy to visualize how a sheet of cells grows, but how does a sheet of cells form a complex, three-dimensional structure? Rebocho et al. describe how differential growth rates between cell layers and across the growing surface can produce a variety of complex shapes. As a model for shape complexity, the authors examine growth patterns in snapdragon (Antirrhinum majus) flowers (wild-type and mutant), and through their analysis along with computational modeling show that “orthogonal directional conflict plays a key role in generating out-of-plane deformations”. They further propose how gene activity, mechanical connectivity and the auxin transporter PIN1 can contribute to the generation of tissue conflicts and therefore shape. Don’t miss the movies! eLIFE  There are about 50,000 fungal species that form mycorrhizal associations with about 250,000 plant species. These associations significantly increase plant productivity by increasing nutrient uptake, particularly nitrogen and phosphorus, although with a considerable carbon cost to plants. Van der Heijden et al. review the ecology and evolution of mycorrhizal associations, focusing on their biodiversity and contributions to ecosystem nutrient cycling. The authors stress that although these systems are often studied as one plant / one fungus, they commonly exist as networks of many individuals and species. Finally, the authors describe how mycorrhizal genomics have impacted our understanding of these associations, as well as key unanswered questions. New Phytol.
There are about 50,000 fungal species that form mycorrhizal associations with about 250,000 plant species. These associations significantly increase plant productivity by increasing nutrient uptake, particularly nitrogen and phosphorus, although with a considerable carbon cost to plants. Van der Heijden et al. review the ecology and evolution of mycorrhizal associations, focusing on their biodiversity and contributions to ecosystem nutrient cycling. The authors stress that although these systems are often studied as one plant / one fungus, they commonly exist as networks of many individuals and species. Finally, the authors describe how mycorrhizal genomics have impacted our understanding of these associations, as well as key unanswered questions. New Phytol.  Sixty years ago, the first report of isoprene (C5H8; 2-methyl-1,3-butadiene) emissions from plants was published. Isoprenes are the largest source of non-methane hydrocarbons in Earth’s atmosphere; furthermore, isoprene is reactive in atmospheric chemistry and can be converted into a variety of harmful compounds. Sharkey and Monson provide a historical overview of the development of our understanding of the role and significance of isoprene emissions to plant biology, and highlight areas where uncertainty remains. For example, although light and temperature are correlated with isoprene emission, emission is also affected by long-term growth conditions and stimulated by change. Furthermore, although it is generally true that isoprene can confer protection against abiotic stresses, the relationship does not always hold, and debate continues about the mechanism for such protection. As yet, we don’t know enough to accurately predict which species will be isoprene emitters (about 20% of perennial plants emit isoprenes), nor the timing and amount of isoprene emissions, nor how global change will impact isoprene emissions. Although much has been learned in the past 60 years, “the biology is still enigmatic”. Plant Cell Environ.
Sixty years ago, the first report of isoprene (C5H8; 2-methyl-1,3-butadiene) emissions from plants was published. Isoprenes are the largest source of non-methane hydrocarbons in Earth’s atmosphere; furthermore, isoprene is reactive in atmospheric chemistry and can be converted into a variety of harmful compounds. Sharkey and Monson provide a historical overview of the development of our understanding of the role and significance of isoprene emissions to plant biology, and highlight areas where uncertainty remains. For example, although light and temperature are correlated with isoprene emission, emission is also affected by long-term growth conditions and stimulated by change. Furthermore, although it is generally true that isoprene can confer protection against abiotic stresses, the relationship does not always hold, and debate continues about the mechanism for such protection. As yet, we don’t know enough to accurately predict which species will be isoprene emitters (about 20% of perennial plants emit isoprenes), nor the timing and amount of isoprene emissions, nor how global change will impact isoprene emissions. Although much has been learned in the past 60 years, “the biology is still enigmatic”. Plant Cell Environ. 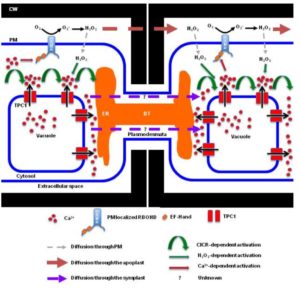 There is ample evidence for rapid, long-distance communication within plants, but our understanding of how these signals are transmitted is incomplete. Choi et al. review recent advances in intercellular signal propagation via Ca2+, reactive oxygen species (ROS) and electrical signals; these “fast” signals are contrasted to the “slow” signals such as jasmonates that accumulate and propagate much more slowly. They address questions such as: How do signals move quickly between cells (~400 – >1000 μm/s)? Do they travel through the apoplast or symplast, and how do plasmodesmata contribute to signal travel? What effect does the cell wall have on signal movement? Which tissues do long-distance signals move through? How are signals decoded, and how do the three signals interact? Plant J.
There is ample evidence for rapid, long-distance communication within plants, but our understanding of how these signals are transmitted is incomplete. Choi et al. review recent advances in intercellular signal propagation via Ca2+, reactive oxygen species (ROS) and electrical signals; these “fast” signals are contrasted to the “slow” signals such as jasmonates that accumulate and propagate much more slowly. They address questions such as: How do signals move quickly between cells (~400 – >1000 μm/s)? Do they travel through the apoplast or symplast, and how do plasmodesmata contribute to signal travel? What effect does the cell wall have on signal movement? Which tissues do long-distance signals move through? How are signals decoded, and how do the three signals interact? Plant J.