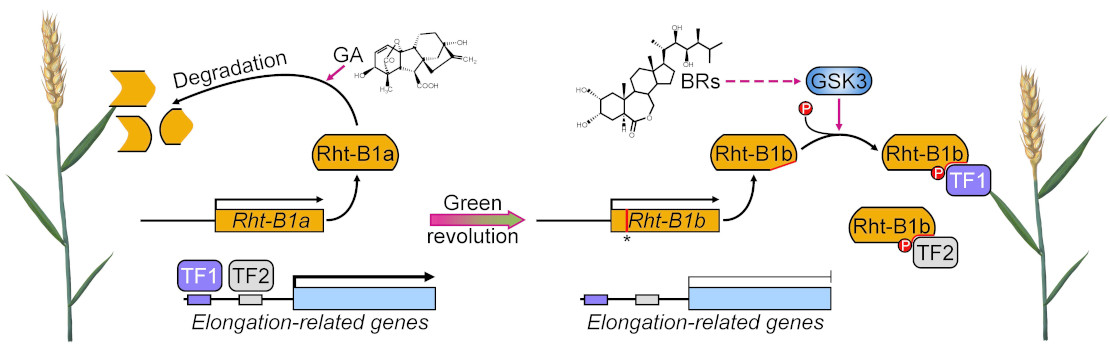
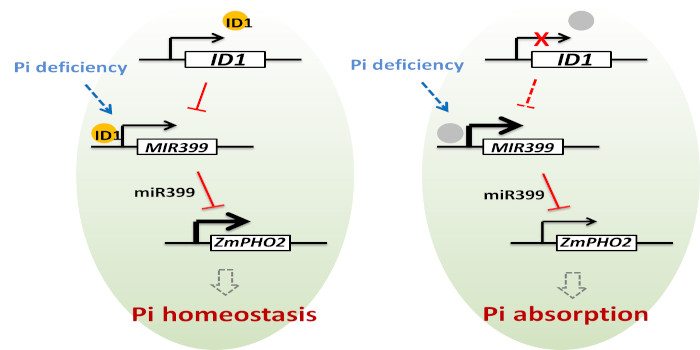
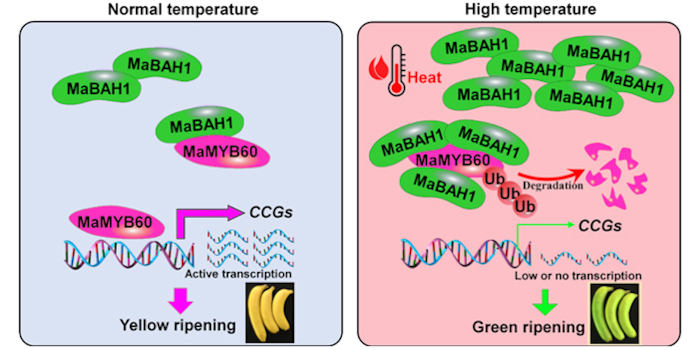
Transmembrane signaling in arbuscular mycorrhizal symbiosis
By Marcel Bucher (University of Cologne) and Li Xue (Zhejiang Normal University)
Background: More than 80% of terrestrial plants form mutualistic symbiosis with arbuscular mycorrhizal (AM) fungi. Arbuscules, the branching hyphae inside the plant cell, are characteristic of AM symbiosis and facilitate nutrient exchange between host and fungi. Arbuscule development is tightly controlled in host cells. Kinases play a central role in cell signaling processes, but the function of AM-induced kinases (AMKs) in arbuscule formation is still largely unknown.
Question: We were interested in identifying AMKs that regulate arbuscule development and deciphering the underlying molecular mechanism.
Findings: Our phylogenetic analysis revealed that nine AMKs are AM-host specific. Among them, the receptor-like kinase KINASE3 (KIN3) and the receptor-like cytoplasmic kinases AMK8 and AMK24 are required for AM symbiosis in Lotus japonicus and are transcriptionally regulated by the transcription factor CTTC MOTIF-BINDING TRANSCRIPTION FACTOR1 (CBX1). AMK8 and AMK24 interact with KIN3 at the cellular membrane. KIN3 and AMK24 are active kinases and AMK24 phosphorylates KIN3. Moreover, the AMK8 and AMK24 homolog in rice is indispensable for AM symbiosis. Our results highlight the critical role of the KIN3-AMK8/24 complex in AM symbiosis and suggest a pathway across the periarbuscular membrane that controls arbuscule development.
Next steps: We plan to identify interacting proteins of the KIN3-AMK8/AMK24 complex that mediate downstream AM signaling, as well as the potential fungal or plant signals that the complex senses.
Reference:
Junchen Leng, Xiaotong Wei, Xinyi Jin, Longxiang Wang, Kai Fan, Ke Zou, Zichao Zheng, Georgios Saridis, Ningkang Zhao, Dan Zhou, Deqiang Duanmu, Ertao Wang, Haitao Cui, Marcel Bucher and Li Xue (2023). ARBUSCULAR MYCORRHIZA-INDUCED KINASES AMK8 and AMK24 associate with the receptor-like kinase KINASE3 to regulate arbuscular mycorrhizal symbiosis in Lotus japonicus. https://doi.org/10.1093/plcell/koad050



 Huijie Liu, co-first author of
Huijie Liu, co-first author of