The functional evolution of geranyl diphosphate synthases with different architectures
Shuyan Song and Shan Lu
School of Life Sciences, Nanjing University, Nanjing 210023, China
Background: Terpenoids are a large group of plant natural products that include small, volatile compounds that contribute to plant aroma (e.g., linalool, limonene, pinene), plant hormones (gibberellins, strigolactones, abscisic acid), carotenoids, and massive compounds that constitute natural rubber. Terpenoids are built with the 5-carbon (C5) precursors isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). Plants use IPP and DMAPP at a 1:1 ratio to synthesize geranyl diphosphate (GPP) and 3:1 ratio to synthesize geranylgeranyl diphosphate (GGPP). GPP is a substrate for making volatile monoterpenes, which plants use to adapt to surrounding environments. GGPP is an intermediate for producing carotenoids, chlorophylls, and other compounds critical for plant survival. GGPP synthases (GGPPSs) exist in all plants, but GPP synthases (GPPSs) are rare in plants other than gymnosperms. Angiosperms generally use Type I small subunit proteins (SSUIs) to have GGPPS produce GPP. There are also Type II SSUs (SSUIIs), which enhance the functions of GGPPS. GPPSs, GGPPSs and SSUs share high sequence similarities. GPPSs and GGPPSs can form homodimers, and GGPPS can also form heterodimers with SSUs.
Question: How did angiosperms develop the strategy of using the GGPPS/SSU combination, instead of genuine GGPPSs and GPPSs, to regulate terpenoid metabolism. How did GPPSs and SSUs evolve?
Findings: By analyzing GGPPS homologs from many photosynthetic organisms, we found GGPPS gene family expansion and functional divergence experienced by ancestral nonvascular plants. This allowed independent parallel evolutionary processes to form genuine GPPSs in gymnosperms and SSUIs in angiosperms. We also found that a pair of amino acids determine the product difference between GPPSs and GGPPSs.
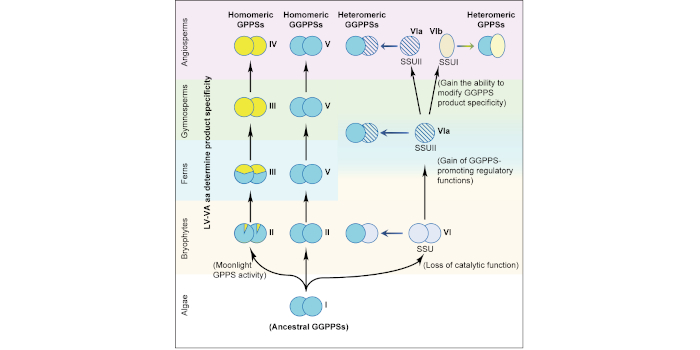
Next steps: At the evolutionary and physiological levels, we want to prove that the GGPPS/SSUI strategy utilized in angiosperms gives plants greater flexibility in modulating terpenoid metabolism, compared with gymnosperms that use separate GGPPS and GPPS, which directly compete for IPP and DMAPP.
Reference:
Shuyan Song, Ruitao Jin, Yufan Chen, Sitong He, Kui Li, Qian Tang, Qi Wang, Linjuan Wang, Mengjuan Kong, Natalia Dudareva, Brian J. Smith, Fei Zhou, Shan Lu. (2023). The functional evolution of architecturally different plant geranyl diphosphate synthases from geranylgeranyl diphosphate synthase. https://doi.org/10.1093/plcell/koad083


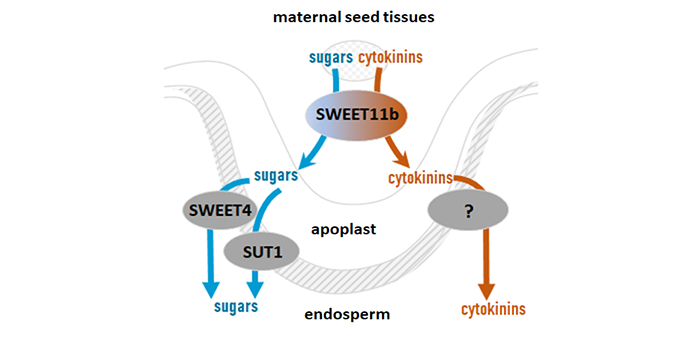 Next steps: This study highlights the relevance of SWEET proteins as multifunctional transporters. It would be of great interest to explore the molecular mechanisms of how cytokinin transport and metabolism towards and in the grains influence their development.
Next steps: This study highlights the relevance of SWEET proteins as multifunctional transporters. It would be of great interest to explore the molecular mechanisms of how cytokinin transport and metabolism towards and in the grains influence their development.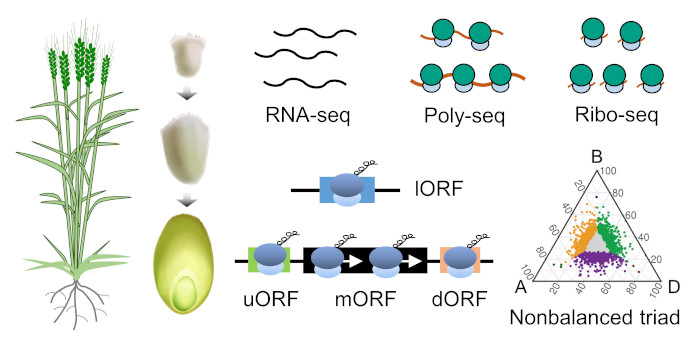 Next steps: Our work presents valuable translatomic resources for understanding the translational control of gene expression during wheat grain development. Further studies will explore functional translational regulatory elements to improve wheat yield and quality.
Next steps: Our work presents valuable translatomic resources for understanding the translational control of gene expression during wheat grain development. Further studies will explore functional translational regulatory elements to improve wheat yield and quality.