A calcium-dependent protein kinase and an E3 ubiquitin ligase fine-tune rice immunity
A calcium-dependent protein kinase and an E3 ubiquitin ligase fine-tune rice immunity
Mou et al. explore the growth–defense trade-off in rice.
https://doi.org/10.1093/plcell/koad265
Baohui Mou, Jiyang Wang, and Wenxian Sun
China Agricultural University
Background: Plants need to keep a balance between growth and defense against diseases. How plants fine-tune this trade-off remains largely a mystery. We identified two protein components in rice disease resistance, a calcium-dependent protein kinase OsCPK17 and an E3 ubiquitin ligase OsPUB12. OsCPK17 promotes accumulation of another immune protein, OsRLCK176, and helps rice plants fight off various diseases. On the other hand, OsPUB12 breaks down OsRLCK176 to prevent excessive immunity. We wanted to understand how the two immune regulators, OsCPK17 and OsPUB12, work together to keep rice plants robust and healthy.
Question: We first aimed to answer the following question: How does the calcium-dependent protein kinase OsCPK17 regulate rice defenses against pathogen attacks? To answer this question, we identified two other proteins, OsRLCK176 and OsPUB12, through protein–protein interaction screening. Next, we asked: How do these proteins work together to control disease resistance in rice?
Findings: In rice, OsRLCK176 is a central player in disease resistance. To balance growth and defense in rice, OsRLCK176 is under tight control by different immune components. We revealed that a calcium-dependent protein kinase OsCPK17 functions as a “boost” button in the rice immune system. When a pathogen threat appears, OsCPK17 is activated to promote OsRLCK176 accumulation and thereby enhance basal defense in rice. However, without danger signals, the E3 ubiquitin ligase OsPUB12 targets OsRLCK176 for recycling, thus keeping basal defense low. We reveal that OsCPK17, OsPUB12 and OsRLCK176 function as a team to fine-tune trade-offs between rice growth and defense.
Next steps: The calcium-dependent protein kinases OsCPK17 and OsCPK4 function as positive and negative regulators through interacting with OsRLCK176, respectively. Future research should examine the molecular mechanisms by which the two calcium sensors function in seemingly opposite ways in rice resistance and aim to create disease-resistant rice plants based on the uncovered immune mechanisms.
Baohui Mou, Guosheng Zhao, Jiyang Wang, Shanzhi Wang, Feng He, Yuese Ning, Dayong Li, Xinhang Zheng, Fuhao Cui, Fang Xue, Shiyong Zhang, Wenxian Sun. (2023). The OsCPK17-OsPUB12-OsRLCK176 module regulates immune homeostasis in rice. https://doi.org/10.1093/plcell/koad265


 Next steps: Future studies will aim to understand the mechanisms underlying the formation of enlarged multivesicular bodies with increased intraluminal vesicles in drif1 drif2 double mutants, and how DRIF regulate SORTING NEXIN 1-dependent membrane protein recycling to the plasma membrane in response to multiple environmental cues.
Next steps: Future studies will aim to understand the mechanisms underlying the formation of enlarged multivesicular bodies with increased intraluminal vesicles in drif1 drif2 double mutants, and how DRIF regulate SORTING NEXIN 1-dependent membrane protein recycling to the plasma membrane in response to multiple environmental cues.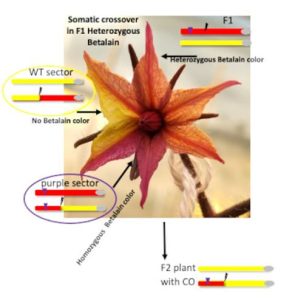 Findings: We confirmed that somatic crossover can be detected visually at a CRISPR-mediated DSB site, opening the prospect for precise breeding in crops. Moreover, we showed that loss of heterozygosity could be due to major chromosomal rearrangements triggered by defective repair of the DSB. Plants where this occurred contained large deletions and translocations and were sterile, showing micronuclei as well as bridges in dicentric chromosomes, at meiosis, as described in McClintock’s Breakage-Fusion-Bridge Cycle triggered by transposons. This genomic reshuffling is similar to chromothripsis in mammalian cells. Both crossover and chromothripsis events were rare but could be detected thanks to the visual assay.
Findings: We confirmed that somatic crossover can be detected visually at a CRISPR-mediated DSB site, opening the prospect for precise breeding in crops. Moreover, we showed that loss of heterozygosity could be due to major chromosomal rearrangements triggered by defective repair of the DSB. Plants where this occurred contained large deletions and translocations and were sterile, showing micronuclei as well as bridges in dicentric chromosomes, at meiosis, as described in McClintock’s Breakage-Fusion-Bridge Cycle triggered by transposons. This genomic reshuffling is similar to chromothripsis in mammalian cells. Both crossover and chromothripsis events were rare but could be detected thanks to the visual assay.