Moving on Up: An MCTP-SNARE Complex Mediates Long-distance Florigen Transport
Flowering plants integrate endogenous and external cues to accurately time the transition from vegetative to reproductive growth (Cho et al., 2017). Many plants, including Arabidopsis thaliana, sense changes in day length (photoperiod) to transition to flowering as the season changes. Decades of careful molecular genetic dissections have uncovered the photoperiod-induced flowering pathway of Arabidopsis, which is centered on sensing increases in day length within leaf phloem cells to activate the expression and transport of phloem-mobile florigen (FT, FLOWERING LOCUS T) to the shoot apical meristem to trigger the floral transition (reviewed in Song et al., 2015).
The underlying molecular mechanisms controlling FT movement from phloem companion cells (CCs) to sieve elements (SEs) for access to the phloem translocation stream are not well understood. To address this issue, Liu et al. (2019) genetically interrogated membrane-resident SNARE (soluble N-ethylmaleimide-sensitive factor protein attachment protein receptor) proteins controlling endosomal vesicular trafficking for potential roles in regulating the CC-to-SE movement of FT during photoperiod flowering in Arabidopsis. The authors identified a late-flowering phenotype exclusively in syp121-4 (syntaxin of plants 121) mutants under long-day conditions that was recapitulated by artificial microRNA-mediated SYP121 knockdowns in wild-type plants and complemented by transforming a genomic gSYP121 fragment back into syp121-4.
 To understand how SYP121 contributes to photoperiod-induced flowering, the authors drew from knowledge of animal syntaxin function and performed yeast 2-hybrid interaction screens against truncated MCTPs (Multiple C2 domain-containing Transmembrane Proteins) lacking transmembrane domains. Surprisingly, this analysis revealed a specific interaction with QKY rather than FTIP1 (FT-INTERACTING PROTEIN1), an MCTP previously implicated in FT movement through the endoplasmic reticulum (Liu et al., 2012). Phenotypic analysis of qky mutants and knockdown lines demonstrated delayed flowering time in long days relative to wild-type plants (Liu et al., 2019). Expression analysis using promoter-reporter fusions revealed that QKY expression is restricted to phloem CCs, and subcellular localization studies determined that QKY-GFP is located in endosomal vesicles and co-localizes with RFP-SYP121 at the plasma membrane. Collectively, these data suggest that a SYP121-QKY complex regulates photoperiod-induced flowering.
To understand how SYP121 contributes to photoperiod-induced flowering, the authors drew from knowledge of animal syntaxin function and performed yeast 2-hybrid interaction screens against truncated MCTPs (Multiple C2 domain-containing Transmembrane Proteins) lacking transmembrane domains. Surprisingly, this analysis revealed a specific interaction with QKY rather than FTIP1 (FT-INTERACTING PROTEIN1), an MCTP previously implicated in FT movement through the endoplasmic reticulum (Liu et al., 2012). Phenotypic analysis of qky mutants and knockdown lines demonstrated delayed flowering time in long days relative to wild-type plants (Liu et al., 2019). Expression analysis using promoter-reporter fusions revealed that QKY expression is restricted to phloem CCs, and subcellular localization studies determined that QKY-GFP is located in endosomal vesicles and co-localizes with RFP-SYP121 at the plasma membrane. Collectively, these data suggest that a SYP121-QKY complex regulates photoperiod-induced flowering.
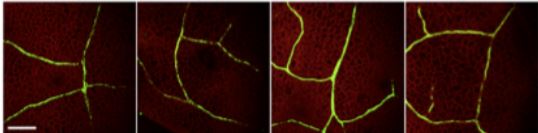
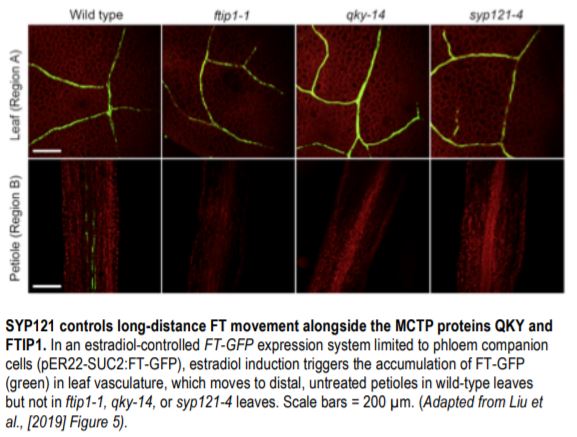
Next, the authors addressed whether QKY interacts with FT to regulate its transport from CCs-to-SEs during photoperiod-induced flowering. As anticipated, protein-protein interaction assays confirmed that QKY interacts with FT, much like the MCTP protein FTIP1 (Liu et al., 2012). To assess the extent to which QKY, SYP121, and FTIP1 contribute to FT movement, the authors performed elegant FT transport assays in the wild type and mutant backgrounds. Using an estradiol-inducible FT-GFP expression system limited to CCs (pER22-SUC2:FT-GFP), the authors demonstrated that estradiol treatment in wild-type leaves led to the accumulation and transport of FT-GFP in distally located untreated petioles. By contrast, FT-GFP was not detected in ftip1, qky, or syp121 petioles after estradiol induction in leaves (see Figure). Similarly, immunogold electron microscopy of plants transformed with SUC2:FT-9myc revealed FT in CCs and SEs in the wild-type background but in CCs and not SEs in the ftip1 qky, syp121 qky, and ftip1 qky syp121 backgrounds. Therefore, a QKY-SYP121 complex mediates FT movement from CCs to SEs through endosomal vesicular trafficking to the plasma membrane, which occurs alongside the FTIP1-mediated transport of FT through the endoplasmic reticulum. Together, these parallel mechanisms ensure that FT is transported long-distance through the phloem to induce the floral transition.
Philip Carella
Sainsbury Laboratory
University of Cambridge,
ORCID: 0000-0002-5467-7290
REFERENCES
Cho L-H, Yoon J and An G (2017). The control of flowering time by environmental factors. Plant J. 90: 708-719.
Liu L, Liu C, Hou X, Xi W, Shen L, Tao Z, Wang Y and Yu H (2012). FTIP1 is an essential regulator required for florigen transport. PLoS Biology 10: e1001313
Liu L, Li C, Teo ZWN, Zhang B and Yu H (2019). The MCTP-SNARE complex regulates florigen transport in Arabidopsis. Plant Cell Published August 2019 DOI: https://doi.org/10.1105/tpc.18.00960
Song YH, Shim JS, Kinmonth-Schultz HA and Imaizumi T (2015). Photoperiodic flowering: time measurement mechanisms in leaves. Ann. Rev. Plant Biol. 66: 441-464.