Microtubules Direct Lignin and Xylan Deposition in a Cellulose-Independent Manner
Although secondary cell walls represent the bulk of plant biomass, the mechanism by which cellulose, hemicellulose, and lignin assemble into a functional three-dimensional matrix is unknown. Cortical microtubules are thought to guide cellulose deposition in the plasma membrane by defining the trajectories of the cellulose synthase complex (CSC; Paradez et al., 2006), whereas hemicelluloses and lignin monomers are produced in the Golgi complex and secreted via vesicular trafficking. Hemicelluloses interact with cellulose microfibrils, forming a stable network, and lignin interacts with hemicelluloses. According to the self-perpetuating cascade model, the localization of cellulose in the secondary cell wall determines the patterning of other cell wall biopolymers (Taylor and Haigler, 1993).
In a Breakthrough Report, Takenaka et al. (2018) provide important insights into secondary cell wall deposition. Using VASCULAR-RELATED NAC-DOMAIN 7 (VND7)-inducible Arabidopsis thaliana lines (VND7-VP16-GR), which undergo ectopic protoxylem vessel cell differentiation following dexamethasone (DEX) treatment, the authors conducted a forward genetic screen for mutants with defects in secondary cell wall patterning. They identified the baculites mutant and showed that it harbored a single nucleic acid substitution in a CSC component named CELLULOSE SYNTHASE SUBUNIT 7 (CESA7) that resulted in loss of CESA7 production.
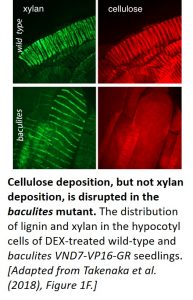 The team then compared the patterning of cellulose, xylan (a hemicellulose), and lignin in the vessel cells of DEX-treated wild-type and baculites VND7-VP16-GR seedlings. Following fixation, cellulose and xylan were detected by confocal microscopy using Pontamine Fast Scarlet 4B and anti-xylan antibody LM10, respectively (see figure), whereas lignin was visualized using multiphoton microscopy. All three biopolymers displayed a helical pattern of distribution in the secondary cell walls of wild-type plants. Even though cellulose deposition was disrupted in the baculites plants, the distribution of xylan and lignin was unaffected, demonstrating that the patterning of these biopolymers does not rely on cellulose localization. Time-lapse analysis showed that strong, linear xylan signals were absent in the mutant at later stages of protoxylem vessel development. Thus, while cellulose is not required for the helical deposition of xylan, it appears to be required for the continued organization of xylan.
The team then compared the patterning of cellulose, xylan (a hemicellulose), and lignin in the vessel cells of DEX-treated wild-type and baculites VND7-VP16-GR seedlings. Following fixation, cellulose and xylan were detected by confocal microscopy using Pontamine Fast Scarlet 4B and anti-xylan antibody LM10, respectively (see figure), whereas lignin was visualized using multiphoton microscopy. All three biopolymers displayed a helical pattern of distribution in the secondary cell walls of wild-type plants. Even though cellulose deposition was disrupted in the baculites plants, the distribution of xylan and lignin was unaffected, demonstrating that the patterning of these biopolymers does not rely on cellulose localization. Time-lapse analysis showed that strong, linear xylan signals were absent in the mutant at later stages of protoxylem vessel development. Thus, while cellulose is not required for the helical deposition of xylan, it appears to be required for the continued organization of xylan.
Given that microtubule rearrangement precedes secondary cell wall development (Oda et al., 2005), the authors next investigated the effect of disrupting the microtubule array on the patterning of each of the cell wall biopolymers. Treatment with the microtubule-depolymerizing drug oryzalin disrupted the helical distribution of xylan and lignin in baculites VND7-VP16-GR seedlings, revealing that the cellulose-independent deposition of these secondary cell wall components is directed by cortical microtubules.
The finding that xylan and lignin deposition continue in the secondary cell wall in the absence of normal cellulose deposition challenges the self-perpetuating cascade model. More studies using the baculites mutant are planned to further examine the mechanism underlying secondary cell wall deposition.
References
Oda Y, Fukuda H. 2012. Initiation of cell wall pattern by a rho- and microtubule-driven symmetry breaking. Science 337: 1333–1336.
Paredez AR, Somerville CR, Ehrhardt DW. 2006. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312: 1491–1495.
Takenaka, Y., Watanabe, Y., Schuetz, M., Unda, F., Hill, J.L. Jr, Phookaew, P., Yoneda, A., Mansfield, S.D., Samuels, L., Ohtani, M., and Demura, T. (2018). Patterned Deposition of Xylan and Lignin is Independent from that of the Secondary Wall Cellulose of Arabidopsis Xylem Vessels. Plant Cell; DOI: 10.1105/tpc.18.00292.
Taylor JG, Haigler CH. 1993. Patterned secondary cell wall assembly in tracheary elements occurs in self-perpetuating cascade. Acta Botanica Neerlandica 42: 153–163.



