KETCHing up with gametogenesis: nucleocytoplasmic import and cell cycle control
In eukaryotes, active import of large signaling molecules and proteins over 40kD in the nucleus is mediated by aptly named “importin” proteins. As there are considerably fewer importins than there are cargos, determining how cargo-transporter specificity is mediated constitutes a challenging issue in the field.
 In Arabidopsis, KETCH1 encodes an essential importin responsible for transporting HYL1, a miRNA processor, to the nucleus (Zhang et al., 2017). However, fertility defects resulting from the loss of KETCH1 suggested additional functions. Xiong and colleagues now report on new functions of KETCH1 (Xiong et al., 2020). Re-examining the loss of function allele ketch1-2/+, Xiong and colleagues found that seed abortion was close to 50%.
In Arabidopsis, KETCH1 encodes an essential importin responsible for transporting HYL1, a miRNA processor, to the nucleus (Zhang et al., 2017). However, fertility defects resulting from the loss of KETCH1 suggested additional functions. Xiong and colleagues now report on new functions of KETCH1 (Xiong et al., 2020). Re-examining the loss of function allele ketch1-2/+, Xiong and colleagues found that seed abortion was close to 50%.
In flowering plants, meiosis produces haploid spores that continue to divide mitotically to produce multicellular organs called gametophytes in which gametes are ultimately formed. The female gametophyte is the embryo sac, and the male gametophyte is the pollen grain. Aborted seeds ratio in ketch1-2/+ plants strongly suggested gametophytic lethality. Indeed, most mutant embryo sacs and pollen grains were unable to develop to maturity and arrested at the first post-meiotic mitotic division. Expression analysis using KETCH1 promoter to drive a nuclear-localized YFP fusion protein revealed KETCH1 was expressed throughout embryo sac development, and during early stages of pollen grain development.
An attractive hypothesis was that KETCH1 was also responsible for nuclear import of HYL1 during gametophyte development. Loss of HYL1 results in a low level of sterility (Zhang et al., 2017). However, Xiong and colleagues did not find evidence of gametophytic sterility in a hyl1 mutant, ruling out that hypothesis.
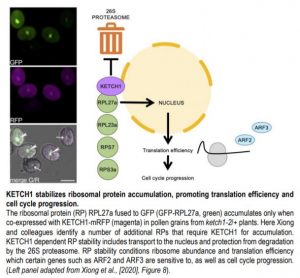
The team then proceeded to identify other cargos of KETCH1. The high similarity of KETCH1 to mammalian IPO5, which imports ribosomal proteins (RPs), suggested KETCH1 might function similarly. Using BiFC and in-vitro pull downs, Xiong and colleagues found that KETCH1 could interact with ribosomal proteins RPL23A, RPS7, RPS3A, RPL27a but NOT RPL5 and RPL23. Interestingly, RPL27a has been shown to be required for embryo patterning and female gametogenesis (Zsögön et al., 2014). Xiong and colleagues further demonstrate this interaction is specific, as a closely related importin, IMB4, did not interact with RPL27a.
To validate this interaction in vivo, Xiong and colleagues introduced GFP-RPL27a and KETCH1-RFP protein fusions driven by the ubiquitous UBQ10 promoter in WT and ketch1-2/+ plants. In ketch1-2 pollen and WT roots, accumulation of GFP-RPL27a signal depended on co-expression of KETCH1-mRFP. GFP-RPL27a also accumulated in response to treatment with the 26S proteasome inhibitor MG132 in roots, suggesting that RPL27a is actively degraded in absence of KETCH1.
To further understand the consequences of reduced stability of RPs, Xiong and colleagues generated plants in which KETCH1 was constitutively knocked-down by a miRNA driven by the 35S promoter (Pro35S::amiR-KETCH1). In these plants, accumulation of GFP-RLP27a was compromised in leaf protoplasts and pavement cells. Polysome profiling further revealed these plants had significantly reduced amounts of 40S and 80S ribosomes, consistent with decreased RP stability.
Would this reduction in ribosome affect protein translation efficiency? To test this, Xiong and colleagues generated GFP fusions with AUXIN RESPONSIVE FACTORS ARF2 and ARF3, which are sensitive to translation efficiency due to an upstream ORF (uORF) in their 5’ leader sequence. GFP-ARF2 and GFP-ARF3 signals were reduced in Pro35S::amiR-KETCH1 plants compared to WT, in an uORF dependent manner. Reduced translation efficiency can result in cell cycle progression defects in yeast and mammals (Donati et al., 2012), and loss of KETCH1 resulted in mitotic arrest during gametogenesis. The authors further examined Pro35S::amiR-KETCH1 leaves, and found these had fewer and larger pavement cells compared to WT. In addition, DNA content profiling revealed increased frequency of pavement cells with 4C and 8C contents compared to WT.
Overall, Xiong and colleagues demonstrate that KETCH1 functions independently of HYL1 in gametophytes, identified RPs as cargos of KETCH1, and uncovered a link between translation efficiency and cell cycle progression. These results provide a framework to further understand transporter-cargo specificity in plants.
Sebastien Andreuzza, DBT-Cambridge Lecturer
Department of Plant Sciences, University of Cambridge, United Kingdom
Center for Cellular and Molecular Biology, Hyderabad, India
ORCID: 0000-0002-5547-2692
REFERENCES
Donati, G., Montanaro, L., and Derenzini, M. (2012). Ribosome Biogenesis and Control of Cell Proliferation: p53 Is Not Alone. Cancer Res 72: 1602–1607.
Xiong, F., Duan, C.-Y., Liu, H.-H., Wu, J.-H., Zhang, Z.-H., Li, S., and Zhang, Y. (2020). Arabidopsis KETCH1 Is Critical for the Nuclear Accumulation of Ribosomal Proteins and Gametogenesis. Plant Cell DOI: https://doi.org/10.1105/tpc.19.00791
Zhang, Z., Guo, X., Ge, C., Ma, Z., Jiang, M., Li, T., Koiwa, H., Yang, S.W., and Zhang, X. (2017). KETCH1 imports HYL1 to nucleus for miRNA biogenesis in Arabidopsis. PNAS 114: 4011–4016.
Zsögön, A., Szakonyi, D., Shi, X., and Byrne, M.E. (2014). Ribosomal Protein RPL27a Promotes Female Gametophyte Development in a Dose-Dependent Manner. Plant Physiology 165: 1133–1143.



