How to go quiet: CHT7 regulates cell cycle exit in Chlamydomonas (Plant Cell)
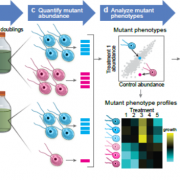
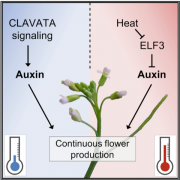
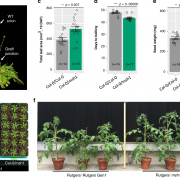
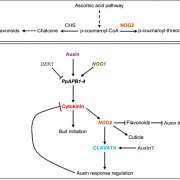
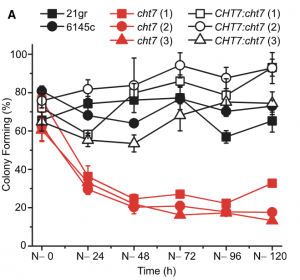 Many microbes enter a state of low metabolic activity, called quiescence, when the conditions for growth and division become unfavorable. The transition from active proliferation to quiescence involves a comprehensive restructuring of cellular metabolism, accumulation of storage compounds, and an orderly exit from the cell cycle. This kind of proliferation-to-quiescence switch has been extensively studied in Chlamydomonas cells that are starved for nitrogen. However, the emphasis in these studies has often been on the starvation-induced accumulation of lipid-based storage compounds, due to their potential industrial uses. Here, Takeuchi et al. instead shift the focus by investigating how the cells cease dividing and exit the cell cycle when nitrogen starts to run out. They show that in the absence of the protein CHT7, the cells keep on dividing and, as a result, rapidly loose viability when nitrogen is withdrawn. Genetically, CHT7 acts as a transcriptional repressor of a broad range of core cell cycle genes in response to nitrogen stress. The underlying mechanism is still an open question, however, and CHT7 itself appears not to bind DNA directly. This study provides a solid foundation for further investigations of the CHT7 pathway, and the coordination between proliferation and quiescence in photosynthetic eukaryotes. (Summary by Frej Tulin @FrejTulin) Plant Cell 10.1105/tpc.19.00628
Many microbes enter a state of low metabolic activity, called quiescence, when the conditions for growth and division become unfavorable. The transition from active proliferation to quiescence involves a comprehensive restructuring of cellular metabolism, accumulation of storage compounds, and an orderly exit from the cell cycle. This kind of proliferation-to-quiescence switch has been extensively studied in Chlamydomonas cells that are starved for nitrogen. However, the emphasis in these studies has often been on the starvation-induced accumulation of lipid-based storage compounds, due to their potential industrial uses. Here, Takeuchi et al. instead shift the focus by investigating how the cells cease dividing and exit the cell cycle when nitrogen starts to run out. They show that in the absence of the protein CHT7, the cells keep on dividing and, as a result, rapidly loose viability when nitrogen is withdrawn. Genetically, CHT7 acts as a transcriptional repressor of a broad range of core cell cycle genes in response to nitrogen stress. The underlying mechanism is still an open question, however, and CHT7 itself appears not to bind DNA directly. This study provides a solid foundation for further investigations of the CHT7 pathway, and the coordination between proliferation and quiescence in photosynthetic eukaryotes. (Summary by Frej Tulin @FrejTulin) Plant Cell 10.1105/tpc.19.00628