Gains in Grain Yield: A Pair of Spikelets Makes All the Difference, Even When One is Sterile
Mother Nature has a way of keeping seemingly useless structures around millions of years. Such structures are likely to have a use that is not obvious, although they could also be remnants of the evolutionary past without extant function, or non-functional but harmless byproducts of a different adaptive feature. In a fascinating study, first authors Taylor AuBuchon-Elder and Viktoriya Coneva and their coworkers (AuBuchon-Elder, Coneva et al., 2020) report on the sterile spiklelet of sorghum and related grasses, and provide support for the idea that this curious structure has been maintained for over 15 million years because it serves an important function.
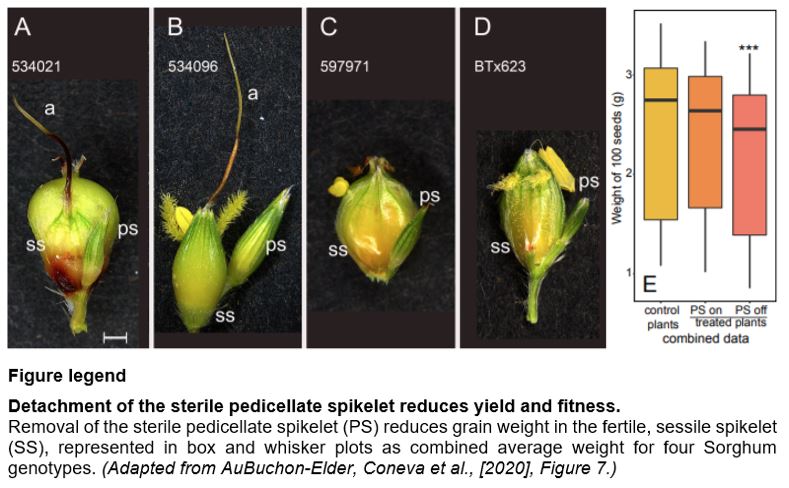 Sorghum belongs to the grass tribe Andropogoneae, where paired spikelets house florets (Kellogg, 2015). In sorghum, a sterile (non-seed bearing) short-stalked pedicellate spikelet (PS) pairs with a fertile, stalkless sessile spikelet (SS) that ultimately bears grain. Additionally, the SS is awned. In wheat, awns can assimilate and transfer carbon to grain (Grundbacher, 1963). PS or awn function in sorghum has not been reported. With these observations and unknowns, the authors set out to understand the significance of the PS and awns in sorghum and related grasses.
Sorghum belongs to the grass tribe Andropogoneae, where paired spikelets house florets (Kellogg, 2015). In sorghum, a sterile (non-seed bearing) short-stalked pedicellate spikelet (PS) pairs with a fertile, stalkless sessile spikelet (SS) that ultimately bears grain. Additionally, the SS is awned. In wheat, awns can assimilate and transfer carbon to grain (Grundbacher, 1963). PS or awn function in sorghum has not been reported. With these observations and unknowns, the authors set out to understand the significance of the PS and awns in sorghum and related grasses.
Could the PS, awn, or both be a source of photosynthate for the SS? To find out, the authors initially conducted a series of 14C labeling and pulse-chase experiments. For 14C labeling, PS had significantly more 14CO2 uptake compared to SS or awns. Additionally, a 24-hour pulse-chase experiment in intact panicles showed a decrease in percent 14C in PS and concomitant percent increase in SS. This observation indicated the 14C had translocated from PS to SS. The idea that PS could be a carbon source made sense because the authors observed stomata on the surface of PS, but not on SS or awns. Stomata are epidermal pores where CO2 enters the organ for photosynthesis. The authors found 14C labeling and pulse-chase results, along with appearance of stomata, to be consistent in two species distantly related to sorghum, Themeda triandra and Andropogon schirensis, which nicely broadened the context of their findings.
If the PS is a carbon source, metabolites produced by photosynthesis should be detectable. The authors explored this hypothesis in intact panicles of sorghum and T. triandra with time-course exposure to 13CO2 followed by LC-MS/MS. The authors cast a wide net in seining for metabolites from C4, C3, sucrose, or starch pathways. Consistent with previous 14C labeling, PS had more 13C than SS or awns. Time-course labeling showed the percent of unlabeled isotopologue fractions for individual metabolites of photosynthesis decreased at the early stages of 13CO2 feeding, indicating carbon assimilation. Similarly, metabolites showed 13C enrichment within five minutes of labeling. The authors conducted transcriptomic analysis of leaf, PS, SS, and awns from the two species, and filtered the data for differentially expressed genes encoding central carbon metabolic enzymes. In support, they found PS accumulated transcripts related to photosynthesis; likewise, these genes were downregulated in awns and SS.
PS have features of a carbon source; SS and awns have sink-like characteristics. How might this relationship impact yield? To address this question, the authors utilized four genotypes with diverse spikelet morphology and simply either detached the PS or left it intact on panicles at anthesis. When the panicles reached maturity, seed weights were collected. Importantly, the authors found a significant ~8.8% reduction in yield between controls (intact) and treatment (detached) across all genotypes (see Figure). Across individual genotypes, mean seed weights dropped from 8-13%, also suggesting that genetic variation in this trait might be leveraged to improve yield.
Photosynthetic PS in the Andropogoneae, albeit reduced in size, have not gone unnoticed by Mother Nature. Rather, they are significant structures that increase yield in domesticated sorghum and fitness in its wild relatives. AuBuchon-Elder, Coneva and coworkers (AuBuchon-Elder, Coneva et al., 2020) have provided a solid foundation for future opportunities to improve sorghum. With a reference genome sequence (Paterson et al., 2009) and being amenable to genome engineering through transformation (Sander, 2019), prospects for gains in sorghum yield are far from sterile.
Josh Strable
Plant Biology Section
School of Integrative Plant Science
Cornell University
ORCID: 0000-0002-0260-8285
REFERENCES
AuBuchon-Elder, T., Coneva, V., Goad, D.M., Jenkins, L.M., Yu, Y., Allen, D.K. and Kellogg, E.A. (2020). Sterile Spikelets Contribute to Yield in Sorghum and Related Grasses. Plant Cell. DOI: https://doi.org/10.1105/tpc.20.00424
Grundbacher, F.J. (1963). The Physiological Function of the Cereal Awns. Bot. Rev. 29: 366-381.
Kellogg, E.A. (2015). Poaceae. In K. Kubitzki, ed, Families and Genera of Vascular Plants. Springer, pp. 1-416.
Paterson, A.H, et al. (2009). The Sorghum bicolor Genome and the Diversification of Grasses. Nature 457: 551-556.
Sander, J.D. (2019). Sorghum: Methods and Protocols. In Z.Y. Zhao and J. Dahlberg. eds. Gene Editing in Sorghum Through Agrobacterium. Springer, pp. 155-168.