Formation of xylem vessels: Role of a WRKY transcription factor
Ge et al explore the function of a transcription factor that functions upstream of a master regulator of tracheary element differentiation. Plant Cell https://doi.org/10.1105/tpc.19.00689
By Shating Ge1, Juan Xu2, and Shuqun Zhang3
1 College of Plant Protection, Nanjing Agricultural University, Nanjing, Jiangsu 210095, China.
2 State Key Laboratory of Plant Physiology and Biochemistry, College of Life Sciences, Zhejiang University, Hangzhou, Zhejiang 310058, China.
3 Division of Biochemistry, University of Missouri, Columbia, MO 65211, USA
Background: Land plants evolved xylem vessels, which transport water and mineral nutrients from roots to above-ground portion of the plants. This enabled plants to colonize and flourish on land. Xylem vessels consist of tracheary elements (TEs), a series of interconnected dead cells with perforations on both ends to form a continuous tubular structure. The development of TEs involves cell specification, patterned secondary wall deposition, and programmed cell death to clear the cellular contents. Based on the thickening patterns of the secondary cell wall, we can identify protoxylem vessels, which have annular or spiral patterned secondary cell walls, and metaxylem vessels, which have pitted secondary cell walls. Xylem cell fate is controlled by a multi-layered transcriptional network, and the Arabidopsis transcription factor VASCULAR-RELATED NAC DOMAIN7 (VND7) is a master regulator of protoxylem vessel differentiation.
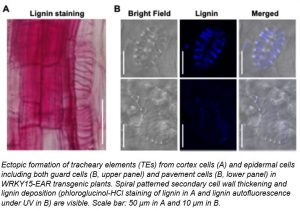 Question: We wanted to understand the biological function(s) of WRKY15, a member of the plant-specific WRKY transcription factor family. Molecular, cellular, and genetic analyses reveal that WRKY15 plays an important function in regulating TE differentiation in Arabidopsis, together with VND7.
Question: We wanted to understand the biological function(s) of WRKY15, a member of the plant-specific WRKY transcription factor family. Molecular, cellular, and genetic analyses reveal that WRKY15 plays an important function in regulating TE differentiation in Arabidopsis, together with VND7.
Findings: We found that overexpression of WRKY15 and WRKY15-EAR, a dominant-negative form of WRKY15, results in opposite phenotypes in TE formation. In WRKY15 overexpressing transgenic seedlings, protoxylem vessels become discontinuous, and the spiral wall thickening of the TEs is reduced. By contrast, expression of the dominant-negative WRKY15-EAR leads to the formation of extra protoxylem vessel files, TEs with an increased spiral wall thickening/lignification, and the transdifferentiation of non-vascular cells (such as cortex cells and hypocotyl/leaf epidermis) into ectopic TE cells. We discovered that WRKY15 suppresses the expression of VND7 based on expression profiling, RT-qPCR, and reporter analyses. The ectopic TE formation in WRKY15-EAR plants is associated with ectopic overexpression of VND7, and the mature ectopic TE cells have undergone complete programmed cell death and cleared their cellular contents, similar to the normal TE differentiation.
Next steps: We are working to find the direct target genes of WRKY15 transcription factor, which will provide further insights into the molecular mechanism underlying the interaction of WRKY15 and VND7 in plant vascular development.
Shating Ge, Xiaofei Han, Xuwen Xu, Yiming Shao, Qiankun Zhu, Yidong Liu, Juan Du, Juan Xu, and Shuqun Zhang (2020) WRKY15 Suppresses Tracheary Element Differentiation Upstream of VND7 During Xylem Formation. Plant Cell https://doi.org/10.1105/tpc.19.00689



