Exo70B2 functions as a exocyst subunit in secretion linked to immunity and autophagy (Plant Cell)
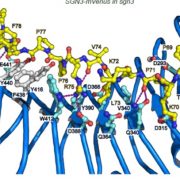
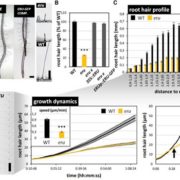
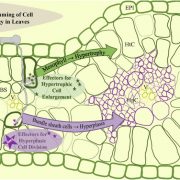
 The exocyst is a conserved protein complex that mediates tethering of secretory vesicle to the plasma membrane prior to SNARE-mediated membrane fusion. Exo70 is one of eight subunits and has expanded into 23 homologs in Arabidopsis. Brillada and Teh et al. identified Exo70B2 as a bona fide exocyst subunit that localize to the plasma membrane and is recruited to the secretory vesicle after being released from the trans-Golgi network. Exo70B2 is transported into the vacuole after treatment with benzothiadiazole (BTH, an analogue of salicylic acid), Concanamycin A and peptide flg22. Transport of Exo70B2 into the vacuole is mediated by autophagy, based on colocalization with autophagosome markers, and confirmed by the absence of Exo70B2 in the vacuole in atg2 mutant. Exo70B2 interacts with and is phosphorylated by MPK3. Phosphorylation mimicking variants of Exo70B2 inhibit localization at sites of active secretion and increase interaction with ATG8 (AUTOPHAGY-RELATED PROTEIN 8), promoting its turnover. Overall, this study sheds light on Exo70B2 functions as a secretion regulator to maintain cellular homeostasis and shows that phosphorylation of this exocyst subunit promotes its degradation, dampening secretory activity. (Summary by Min May Wong @wongminmay) Plant Cell 10.1093/plcell/koaa022/6017825
The exocyst is a conserved protein complex that mediates tethering of secretory vesicle to the plasma membrane prior to SNARE-mediated membrane fusion. Exo70 is one of eight subunits and has expanded into 23 homologs in Arabidopsis. Brillada and Teh et al. identified Exo70B2 as a bona fide exocyst subunit that localize to the plasma membrane and is recruited to the secretory vesicle after being released from the trans-Golgi network. Exo70B2 is transported into the vacuole after treatment with benzothiadiazole (BTH, an analogue of salicylic acid), Concanamycin A and peptide flg22. Transport of Exo70B2 into the vacuole is mediated by autophagy, based on colocalization with autophagosome markers, and confirmed by the absence of Exo70B2 in the vacuole in atg2 mutant. Exo70B2 interacts with and is phosphorylated by MPK3. Phosphorylation mimicking variants of Exo70B2 inhibit localization at sites of active secretion and increase interaction with ATG8 (AUTOPHAGY-RELATED PROTEIN 8), promoting its turnover. Overall, this study sheds light on Exo70B2 functions as a secretion regulator to maintain cellular homeostasis and shows that phosphorylation of this exocyst subunit promotes its degradation, dampening secretory activity. (Summary by Min May Wong @wongminmay) Plant Cell 10.1093/plcell/koaa022/6017825