EMFasizing the conserved function of Polycomb in rice endosperm development
F1 plants resulting from crosses between parents of different strains often exhibit improved fitness, known as heterosis, or also hybrid vigor. Hybrids are heavily used in modern agriculture and could be part of the solution to address the challenge of feeding an ever-growing world population. However, heterosis declines in the F2 and subsequent generations because two key processes inherent to sexual reproduction, meiosis and fertilization, generate homozygous alleles among the progeny.
Our food essentially comes from the seeds produced by sexual reproduction of flowering plants (angiosperms), which include the model Arabidopsis as well as cereals such as rice, the most widely consumed staple food for a large part of the world’s population. These species reproduce exclusively sexually by a process known as double fertilization. Two female gametes are produced in ovules, the egg cell and the central cell, and two male gametes called sperm cells are produced in pollen grains. During fertilization, a sperm cell fuses with the egg cell to produce the embryo and the other sperm cell fuses with the central cell to produce the endosperm. The endosperm supports embryo growth and does not participate to the next generation. However, several angiosperm species can bypass the sexual pathway and produce seeds that are clones of their maternal parent. Asexual seed formation, or apomixis, is considered to be the holy grail of agriculture because it would fix heterosis across generations (Drews and Koltunow, 2011).
Apomixis requires three components: apomeiosis, parthenogenesis, and autonomous endosperm development. Apomeiosis refers to production of gametes without meiosis and parthenogenesis refers to development of the embryo without egg cell fertilization. Apomeiosis and parthenogenesis have recently been combined in rice to obtain progeny from hybrid parents that retained heterosis, but yields remained low (Khanday et al., 2019; Wang et al., 2019). This result could potentially be improved by adding the third component of apomixis, autonomous endosperm development, which refers to endosperm formation without central cell fertilization. In Arabidopsis, mutants in subunits of the FERTILISATION INDEPENDENT SEED (FIS) complex produce unfertilized seeds with autonomous endospermy at high frequency. FIS genes encode homologues of the animal Polycomb repressing complex PRC2, which deposits the repressive mark H3K27me3. FIS comprises four subunits, including the zinc finger protein FERTILISATION INDEPENDENT SEED 2 (FIS2). However, studies of FIS outside Arabidopsis have hardly revealed a conserved function (Drews and Koltunow, 2011).
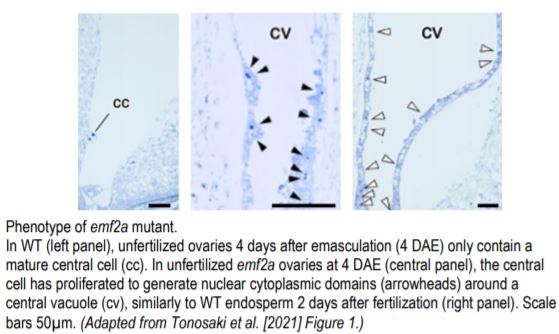 A team led by Kaoru Tonosaki in the group of Tetsu Kinoshita now provides evidence that loss of PRC2 function in the central cell leads to autonomous development of the endosperm in rice (Tonosaki et al,. 2020). The group used CRISPR/Cas9 to generate mutants in EMBRYONIC FLOWER 2a (EMF2a), a putative homologue of FIS2. In ~40% of florets, almost all emf2a ovules enlarged when fertilization was prevented in 2 independent alleles. Microscopic analyses of these ovules revealed presence of developing endosperm but absence of an embryo (see figure). The endosperm in these seeds displayed mature features including nuclear cytoplasmic domains and accumulation of starch granules. Transcript profiling confirmed the unfertilized origin of the endosperm in emf2a enlarged seeds. Another group independently generated emf2a mutants and obtained similar results (Cheng et al., 2020).
A team led by Kaoru Tonosaki in the group of Tetsu Kinoshita now provides evidence that loss of PRC2 function in the central cell leads to autonomous development of the endosperm in rice (Tonosaki et al,. 2020). The group used CRISPR/Cas9 to generate mutants in EMBRYONIC FLOWER 2a (EMF2a), a putative homologue of FIS2. In ~40% of florets, almost all emf2a ovules enlarged when fertilization was prevented in 2 independent alleles. Microscopic analyses of these ovules revealed presence of developing endosperm but absence of an embryo (see figure). The endosperm in these seeds displayed mature features including nuclear cytoplasmic domains and accumulation of starch granules. Transcript profiling confirmed the unfertilized origin of the endosperm in emf2a enlarged seeds. Another group independently generated emf2a mutants and obtained similar results (Cheng et al., 2020).
Tonosaki and colleagues further explored developmental functions of EMF2a in fertilized endosperms. Combining RNA-seq and ChIP-seq in WT and emf2a mutant seeds, the authors found that gene expression was altered and levels of H3K27me3 were decreased in emf2a endosperms, consistent with EMF2a being required for PRC2 function. Correlating reduced H3K27me3 with genes whose expression increased identified putative direct targets of EMF2a. Interestingly, these candidates include MADS-box transcription factors, which are also targets of FIS in Arabidopsis (Batista and Köhler, 2020), further suggesting a conserved function.
In addition to providing strong evidence for conserved PRC2 function in rice endosperm, the work by Tonosaki and colleagues identifies a novel autonomous endosperm development trigger, which combined with strategies that induce apomeiosis and parthenogenesis, could take us closer to engineering apomixis in crops.
Sebastien Andreuzza,
DBT-Cambridge Lecturer
Department of Plant Sciences, University of Cambridge, United Kingdom
Center for Cellular and Molecular Biology, Hyderabad, India
ORCID: 0000-0002-5547-2692
References
Batista, R.A. and Köhler, C. (2020). Genomic imprinting in plants—revisiting existing models. Genes Dev. 34: 24–36.
Cheng, X., Pan, M., E, Z., Zhou, Y., Niu, B., and Chen, C. (2020). The Maternally Expressed Polycomb Group Gene OsEMF2a Is Essential for Endosperm Cellularization and Imprinting in Rice. Plant Communications: 100092.
Drews, G.N. and Koltunow, A.M.G. (2011). The Female Gametophyte. The Arabidopsis Book: e0155.
Khanday, I., Skinner, D., Yang, B., Mercier, R., and Sundaresan, V. (2019). A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 565: 91–95.
Tonosaki, K. et al. (2021). Mutation of the imprinted gene OsEMF2a induces autonomous endosperm development and delayed cellularization in rice. Plant Cell https://bit.ly/33DQdqW
Wang, C., Liu, Q., Shen, Y., Hua, Y., Wang, J., Lin, J., Wu, M., Sun, T., Cheng, Z., Mercier, R., and Wang, K. (2019). Clonal seeds from hybrid rice by simultaneous genome engineering of meiosis and fertilization genes. Nat Biotechnol 37: 283–286.