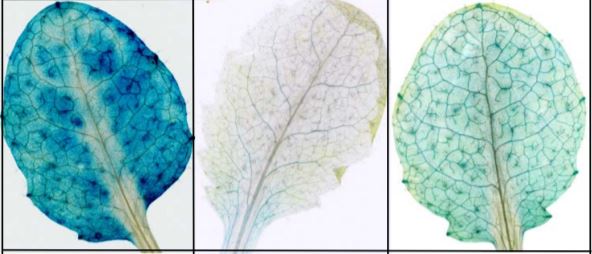
Cutting the mustard: Evolving ER structures into ER bodies for plant defence
The endoplasmic reticulum (ER) has been likened to the manufacturing and packaging department of a factory, an action packed production area that distributes things to the rest of the cell. The ER does this by forming complex networks of tubules and cisternae that connect to other membranous organelles. In certain plant species, specialised ER-derived compartments form in addition to those crucial for normal cellular processes. These structures include protein bodies that accumulate seed storage proteins in maize and rice and protease-accumulating vesicles in germinating seeds of beans. ER bodies are another ER-derived structure only found in the Brassicales. They are continuous with the ER network and form a distinct rod-shaped structure covered in ribosomes. So why have the Brassicales developed such unique ER structures? Mustard bombs! Plants in the Brassicales produce glucosinolates and other secondary metabolites that make the ‘mustard bombs’ involved in plant defence against insects and microbial pathogens. The major component of ER bodies in Arabidopsis is PYK10, a b-glucosidase that was recently shown to hydrolyze indole glucosinolates into products toxic to pathogens and herbivores (Nakano et al., 2017). ER bodies confer a way to segregate PYK10 from its substrates in discrete cellular compartments. ER bodies are found in the cotyledons, hypocotyls and roots of young seedlings and are not present in mature leaf tissues, yet can be induced by wounding or treatment with methyl jasmonate (reviewed in (Nakano et al., 2014). This supports the proposed role of ER bodies in defence and stress responses.
 A key question is how these distinct ER body structures arose? Did they originate in the Brassicales or evolve from related ER structures? Factors essential for ER body formation include NAI1 and NAI2. NAI1 encodes a transcription factor that acts as a master regulator of genes encoding ER body components and NAI2 forms complexes with MEMBRANE OF ER BODY1 (MEB1) and MEB2, integral membrane proteins that accumulate in ER bodies (Yamada et al., 2013). nai2 mutants essentially lack ER bodies and result in PYK10, MEB1 and MEB2 being diffused throughout the ER. Homologs of NAI2 are only found in plants in the Brassicaceae order that form ER bodies, suggesting a specific role for NAI2 in ER body formation (Yamada et al., 2008).
A key question is how these distinct ER body structures arose? Did they originate in the Brassicales or evolve from related ER structures? Factors essential for ER body formation include NAI1 and NAI2. NAI1 encodes a transcription factor that acts as a master regulator of genes encoding ER body components and NAI2 forms complexes with MEMBRANE OF ER BODY1 (MEB1) and MEB2, integral membrane proteins that accumulate in ER bodies (Yamada et al., 2013). nai2 mutants essentially lack ER bodies and result in PYK10, MEB1 and MEB2 being diffused throughout the ER. Homologs of NAI2 are only found in plants in the Brassicaceae order that form ER bodies, suggesting a specific role for NAI2 in ER body formation (Yamada et al., 2008).
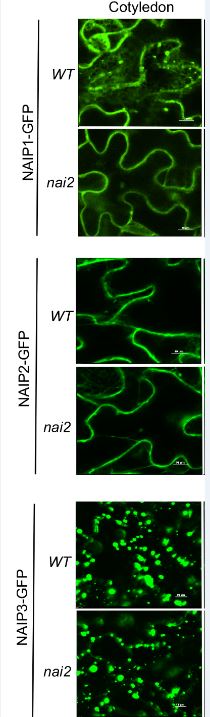
Wang et al., 2019 (this edition) identify three closely related NAI2-interacting proteins (NAIP1, 2, 3) that provide support for ER bodies having evolved in the Brassicales from existing ER structures. A neat set of BiFC experiments in Arabidopsis show that NAI2 and NAIPs interact on the cytosolic face of ER bodies. NAIP-GFP fusions show they are all components of ER bodies. Redundant roles for NAIPs in ER body formation is also shown through naip1 naip2 naip3 triple mutants. So far, so good, this all fits with NAIPs playing a role in ER body formation. Yet NAIPs are not entirely confined to ER bodies. Whereas NAIP1 shows characteristics similar to other ER body components such as PYK10 and MEBs, being largely confined to ER-bodies in a NAI2-dependent manner, NAIP2 and NAIP3 do not. NAIP2 and NAIP3 are also present in other types of ER-derived vesicular structures and in tissues where ER bodies do not form (Figure 1). An elegant set of domain swapping experiments between NAIP1 and NAIP3 revealed the N-terminal domain, the region involved in self- and mutual-interactions, determines tissue specificity and ER compartment location. Unlike NAI2, homologs of NAIP sequences are not restricted to Brassicales and were identified in species throughout the plant lineage and in some protists. This suggests that ER bodies in Brassicales became specialised from pre-existing NAIP-containing ER structures. A number of intriguing questions are raised about the functional roles of NAIPs. For example, do different NAIP-containing ER-structures have roles in defence and stress responses similar to ER bodies? What kinds of molecules do these contain? The sophistication of forming specialised ER-compartments for defence, protein storage and other purposes shows we still have much to learn about these fascinating ER networks.
Figure 1. NAIP-containing ER-derived structures in epidermal cells of Arabidopsis cotyledons in WT and nai2 mutants.
References:
Nakano RT, Pislewska-Bednarek M, Yamada K, Edger PP, Miyahara M, Kondo M, Bottcher C, Mori M, Nishimura M, Schulze-Lefert P, Hara-Nishimura I, Bednarek P (2017) PYK10 myrosinase reveals a functional coordination between endoplasmic reticulum bodies and glucosinolates in Arabidopsis thaliana. Plant J 89: 204-220
Nakano RT, Yamada K, Bednarek P, Nishimura M, Hara-Nishimura I (2014) ER bodies in plants of the Brassicales order: biogenesis and association with innate immunity. Front Plant Sci 5: 73
Yamada K, Nagano AJ, Nishina M, Hara-Nishimura I, Nishimura M (2008) NAI2 is an endoplasmic reticulum body component that enables ER body formation in Arabidopsis thaliana. Plant Cell 20: 2529-2540
Yamada K, Nagano AJ, Nishina M, Hara-Nishimura I, Nishimura M (2013) Identification of two novel endoplasmic reticulum body-specific integral membrane proteins. Plant Physiol 161: 108-120
Wang Z, Li X, Liu N, Peng Q, Wang Y, Fan B, Zhu C, Chen Z (2019) A family of NAI2-interacting proteins in the biogenesis of the ER body and related structures. Plant Physiol (this edition)