Cryptochromes Go Toe to Toe with TOEs Too
Scott Hayes
Affiliation: Laboratory of Plant Physiology, Wageningen University, Wageningen 6708 PB, The Netherlands
ORCiD: 0000-0001-8943-6238
To breed or not to breed, that is the question. The switch from vegetative to reproductive growth is one of the most important steps in a plant’s life cycle. Flower too early or too late and there is a risk that the environment will not support the development of healthy offspring. To avoid this, the timing of flowering is tightly controlled by environment cues. In Arabidopsis thaliana, it is possible to promote the flowering transition by exposing plants to long-day photoperiods.
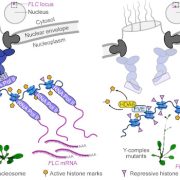
Long photoperiods are detected by the blue light photoreceptor cryptochrome 2 (cry2). For blue light to promote flowering, it must coincide with internal cues. The expression of the floral integrator CONSTANS (CO) peaks around 16 hours after dawn. When photoperiods are short, the peak CO expression occurs in the dark and CO is quickly degraded by the E3-ligase CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1). However, if the expression of CO coincides with light (as occurs in long days), COP1 is inhibited by cry2, allowing CO protein to accumulate. CO induces the expression of FLOWERING TIME (FT) and thereby promotes the transition to reproductive growth (Song et al., 2015) (Fig 1). cry2 also promotes flowering more directly though interaction with the transcription factor cry2 INTERACTING bHLH1 (CIB1). CIB1, CO and cry2 form a complex that accumulates at the FT promoter and enhances FT expression (Song et al., 2015; Liu et al., 2018) (Fig. 1).
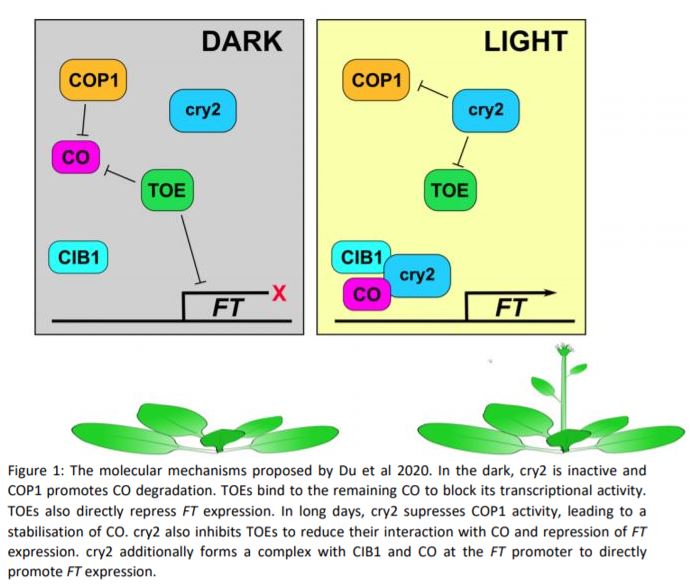 In this issue of Plant Physiology, Shasha Du et al. add yet another string to cry2’s bow. In a screen for cry1 interacting proteins, the group identified TARGET OF EAT 1 (TOE1), an APETALIA 2-like (AP2-like) family transcription factor. TOE1 has previously been shown to control flowering by binding to CO and blocking its activity (Zhang et al., 2015). Because cry2 is the predominant cryptochrome in the regulation of flowering, the group tested whether cry2 also bound to TOE1. Indeed, they found that cry2 binds to TOE1, TOE2 and other members of the AP2-like family. Curiously, the group found that the interaction between cry2 and AP2-like transcription factors occurred in the dark in yeast, but was blue light-dependent in plants. It is unclear why the blue light requirement differs between these two systems.
In this issue of Plant Physiology, Shasha Du et al. add yet another string to cry2’s bow. In a screen for cry1 interacting proteins, the group identified TARGET OF EAT 1 (TOE1), an APETALIA 2-like (AP2-like) family transcription factor. TOE1 has previously been shown to control flowering by binding to CO and blocking its activity (Zhang et al., 2015). Because cry2 is the predominant cryptochrome in the regulation of flowering, the group tested whether cry2 also bound to TOE1. Indeed, they found that cry2 binds to TOE1, TOE2 and other members of the AP2-like family. Curiously, the group found that the interaction between cry2 and AP2-like transcription factors occurred in the dark in yeast, but was blue light-dependent in plants. It is unclear why the blue light requirement differs between these two systems.
To investigate whether cry2:TOE interaction plays a role in flowering regulation, the group created a cry1 cry2 toe1 toe2 quadruple knock-out mutant. These plants flowered slightly earlier than the cry1 cry2 mutant, suggesting that cryptochromes promote flowering at least in part through the inhibition of TOEs. The group also showed that the overexpression of either TOE1 or TOE2 represses flowering much more strongly in the cry1 cry2 mutant. They went on to demonstrate that cry2 blocks the interaction between TOEs and CO in a blue light-dependent manner. They propose that reduced TOE:CO interaction promotes CO activity and allows for flowering induction. The group also established that the interaction between cry2 and TOE1 blocked TOE1 from binding to a specific site 3’ of the FT promoter. They suggest cry2-mediated suppression of TOEs promotes flowering in two ways, both by increasing the pool of functional CO and by releasing direct repression of FT expression by TOEs (Fig 1).
In addition to improving our understanding of cry2-mediated flowering, this study brings up some important questions. It is curious that TOE1 binding to the FT promoter was increased in the cry1 cry2 background at only one of four TOE1 binding sites. If cry2 simply inhibits TOE1 DNA binding, should we not expect all binding sites to be enriched in the absence of cry2? Selectivity in this response hints that cry2-mediated inhibition of TOE1 DNA binding is more nuanced than simply through sequestration. Another aspect that could be further explored is the effect of TOEs on the cry2:CIB1:CO complex. CO is a B-box family transcription factor. Recently it was shown that other members of the B-box family act as rate limiting co-factors for a master regulator of photomorphogenesis, ELONGATED HYPOCOTYL 5 (HY5) (Bursch et al., 2020). If CO acts as the rate-limiting component of the cry2:CIB1:CO multimer, TOE:CO interaction could potentially modulate the transcriptional activity of the complex. Finally, it is currently unclear when cry2 mediates suppression of TOEs. toe1 toe2 mutants flower early in both short days and long days (Zhang et al., 2015), whereas cry2 affects flowering only in long days (Song et al., 2015). This implies that cry2 suppression of TOEs mainly plays a role towards the end of the day. Future research should provide some valuable insights into these questions and improve our understanding of how plants make that important decision of switching to reproductive growth.
LITERATURE CITED
Bursch K, Toledo-Ortiz G, Pireyre M, Lohr M, Braatz C, Johansson H (2020) Identification of BBX proteins as rate-limiting cofactors of HY5. Nat Plants. doi: 10.1038/s41477-020-0725-0
Liu Y, Li X, Ma D, Chen Z, Wang J, Liu H (2018) CIB1 and CO interact to mediate CRY2‐dependent regulation of flowering. EMBO Rep 19: 1–10
Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T (2015) Photoperiodic Flowering: Time Measurement Mechanisms in Leaves. Annu Rev Plant Biol 66: 441–464
Zhang B, Wang L, Zeng L, Zhang C, Ma H (2015) Arabidopsis TOE proteins convey a photoperiodic signal to antagonize CONSTANS and regulate flowering time. Genes Dev 29: 975–987