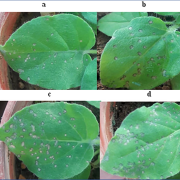
CPK28 is targeted by the ubiquitin ligases ATL31 and ATL6 for proteasome-mediated degradation to fine-tune immune signaling
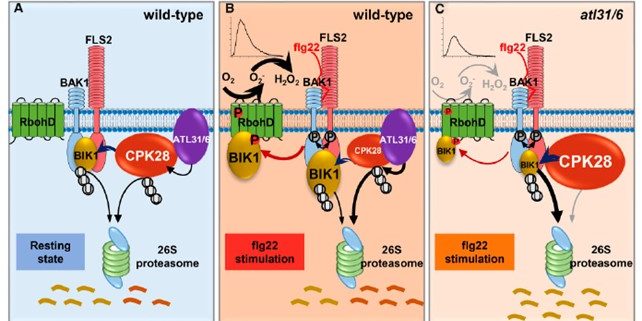
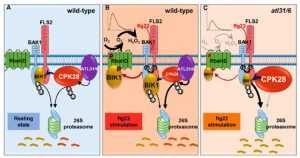 ‘Fine tuning’ is a very significant process that takes place in the cell to correctly modulate plant responses. BOTRYTIS-INDUCED KINASE 1 (BIK1) is an important hub protein that is at the center of the signaling hub that controls defense responses. Hence, plants fine tune this important protein through several processes, including proteasomal degradation mediated by a Ca2+ dependent protein kinase CDPK28 during the absence of stress. In a recent report by Liu et al. in The Plant Cell, the authors show that the CPK28 protein is itself regulated by proteasomal degradation to free up BIK1 to elicit the defense response. Under pathogen attack, which the authors elicited by flagellin 22 (flg22) treatment, two ubiquitin ligases ATL31 and ATL6 interact with CPK28 and to promote its degradation through the 26S proteasome system. This frees up significant amount of BIK1 protein that can now activate RbohD by phosphorylation, leading to ROS production. In separate events, BIK1 can also activate other processes, including the closure of stomata and the initation of the Ca2+ signaling cascade, to further enhance the plant’s defense response. (Summary by Sibaji K Sanyal) Plant Cell 10.1093/plcell/koab242
‘Fine tuning’ is a very significant process that takes place in the cell to correctly modulate plant responses. BOTRYTIS-INDUCED KINASE 1 (BIK1) is an important hub protein that is at the center of the signaling hub that controls defense responses. Hence, plants fine tune this important protein through several processes, including proteasomal degradation mediated by a Ca2+ dependent protein kinase CDPK28 during the absence of stress. In a recent report by Liu et al. in The Plant Cell, the authors show that the CPK28 protein is itself regulated by proteasomal degradation to free up BIK1 to elicit the defense response. Under pathogen attack, which the authors elicited by flagellin 22 (flg22) treatment, two ubiquitin ligases ATL31 and ATL6 interact with CPK28 and to promote its degradation through the 26S proteasome system. This frees up significant amount of BIK1 protein that can now activate RbohD by phosphorylation, leading to ROS production. In separate events, BIK1 can also activate other processes, including the closure of stomata and the initation of the Ca2+ signaling cascade, to further enhance the plant’s defense response. (Summary by Sibaji K Sanyal) Plant Cell 10.1093/plcell/koab242