Calcium signaling in senescence
Durian et al. searched for phosphorylation substrates of Arabidopsis CALCIUM DEPENDENT PROTEIN KINASE1 and found that it phosphorylates ORE1, a master regulator of senescence, demonstrating a link between calcium signaling and cell death. Plant Cell https://doi.org/10.1105/tpc.19.00810
By Guido Durian1 and Tina Romeis2
1University of Turku, Molecular Plant Biology, Department of Biochemistry, FI-20014 Turku, Finland
2Leibniz Institute of Plant Biochemistry, Department Biochemistry of Plant Interactions, 06120 Halle (Saale), Germany
Background: Plant calcium-dependent protein kinases (CDPKs) are enzymes that have a modular protein structure with a dual sensor-effector function. Within the same protein, the input (calcium binding) is recognized by a sensor domain that controls the output (substrate phosphorylation) of a kinase effector domain. CDPKs have been characterized mostly in signaling processes, when, for example, alterations in the external environment by abiotic or biotic stresses lead to increased cytoplasmic calcium concentrations. These concentration changes are sensed and translated by CDPKs to induce tolerance or immune responses. Genomic analyses have described CDPK gene families and their phylogenetic relationships from many plant species and correlations between environmental changes and CDPK gene expression exist. However, in vivo biochemical knowledge about CDPK-phosphorylation target proteins, which transmit the signal downstream of CDPKs in a defined biological context, is rare.
Question: What are the biological phosphorylation substrate proteins of CDPKs? How can these CDPK phosphorylation targets be identified in biological processes that are not yet known to be responsive to changes in the cytoplasmic calcium concentration? How does substrate phosphorylation translate the original calcium signal into the appropriate response of a cell?
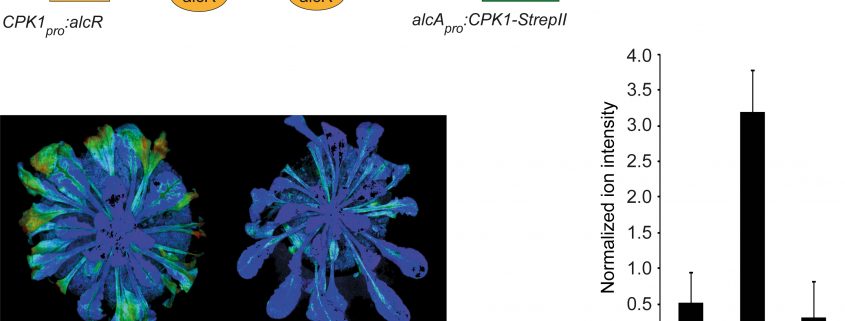
Findings: We screened for differential protein phosphorylation by the calcium-dependent protein kinase CPK1. Using the ethanol-inducible expression of a constitutively active CPK1 variant, phosphorylation of target proteins occurred in the absence of a yet unknown external/endogenous biological stimulus. Phosphoproteomic analysis identified ORE1, a master regulator of cell death in plant senescence, as a phosphorylation substrate of CPK1. The region identified in ORE1 that is phosphorylated by CPK1 is functionally required for ORE1 to function in gene activation. Our data reveal that a biological pathway, senescence, which is known to be strictly controlled by gene-regulatory networks, is subject to an additional layer of control through the calcium-dependent signaling network via CPK1.
Next steps: Because activation of CPK1 protein kinase activity depends on an elevated cytoplasmic calcium level, future research will address the identity of this activating calcium stimulus. It will investigate the source of cytoplasmic calcium concentration changes during the onset of leaf senescence and attempt to identify calcium channels effecting it.
Guido Durian, Mastoureh Sedaghatmehr, Lilian P. Matallana-Ramirez, Silke Schilling, Sieke Schaepe, Tiziana Guerra, Marco Herde, Claus-Peter Witte, Bernd Mueller-Roeber, Waltraud X. Schulze, Salma Balazadeh and Tina Romeis. (2020). Calcium-Dependent Protein Kinase CPK1 Controls Cell Death by In Vivo Phosphorylation of Senescence Master Regulator ORE1. Plant Cell. https://doi.org/10.1105/tpc.19.00810