BSD2 is a Rubisco specific assembly chaperone, forms intermediary hetero‐oligomeric complexes and is non‐limiting to growth in tobacco (Plant Cell Environ)
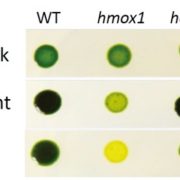
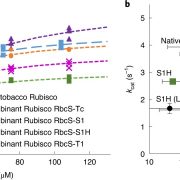
The rubisco holoenzyme is comprised of eight large subunits and eight small subunits (L8S8). Several auxiliary proteins are required to correctly assemble the functional protein. In this manuscript, Conlan et al investigate the chaperone function of one of these proteins, BSD2, in tobacco. The authors confirmed that in leaf tissue, BSD2 forms stable complexes with L8, which may or may not also contain rubisco small subunits. In addition to complexed BSD2, a small amount of uncomplexed protein was also observed, indicating that BSD2 might have additional functions. However, RNAi silencing of BSD2 demonstrated that its only function appeared to be rubisco assembly. BSD2 was specific for Form I rubisco, although BSD2 from tobacco, Arabidopsis and maize were all able to rescue rubisco production in BSD2-RNAi tobacco lines. Over-expression of BSD2 did not lead to increased production of L8S8 rubisco or L8-BSD2, indicating that BSD2 is not normally limiting. The implications of these data for engineering different rubisco isoforms into plants is discussed. (Summary by Mike Page) Plant Cell Environ 10.1111/pce.13473