AvrRpm1, a 28-year Old Mystery Solved
Reddit et al. show that an effector protein from a pathogen modifies a host factor by ADP ribosylation, thereby activating defenses.
The Plant Cell https://doi.org/10.1105/tpc.19.00020
Background:
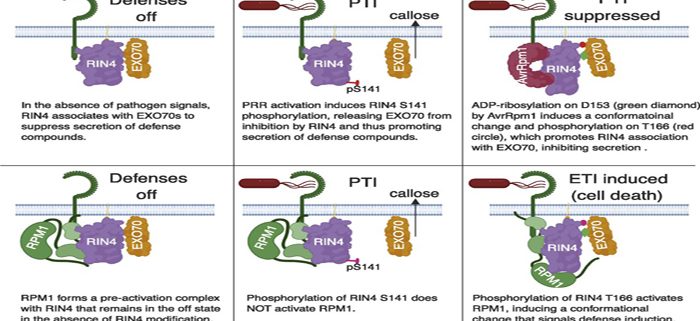
To protect themselves against disease, plants employ a family of intracellular receptors called disease resistance (R) proteins. Most R proteins function by monitoring the status of other plant proteins that are themselves targeted by pathogen proteins. When an R protein detects the modification of one of these target proteins, it sounds the alarm and the plant mounts a defense response. In Arabidopsis, the R protein RPM1 can detect the presence of two different bacterial proteins, AvrB and AvrRpm1, both of which target the same plant protein, RIN4. While it has been known since 2002 that AvrB and AvrRpm1 target RIN4, exactly what they do to RIN4 has remained unclear.
Question:
We wished to determine the precise chemical modification that AvrRpm1 induces on RIN4, how this modification leads to the activation of R-proteins, and how, in the absence of an R-protein-mediated defense response, this modification promotes disease.
Findings:
By transiently overexpressing AvrRpm1 and RIN4 protein together in the leaves of a tobacco relative, Nicotiana benthamiana, we were able to purify large amounts of modified RIN4 protein, which we analyzed using mass spectrometry. These analyses revealed that AvrRpm1 catalyzes the addition of ADP-ribose on two specific amino acids of RIN4, both of which reside within so-called ‘nitrate-induced (NOI) domains’. RIN4 contains two NOI domains, and prior work had shown that phosphorylation of threonine 166 within the C-terminal NOI domain of Arabidopsis RIN4 can activate RPM1. Our studies revealed that the addition of ADP-ribose on aspartate 153 promotes phosphorylation on T166, thus explaining how AvrRpm1 activates RPM1. Our studies also revealed that the C-terminal NOI domain mediates the association of RIN4 with EXO70, a protein involved in secretion. In the absence of RPM1, we speculate that ADP-ribosylation of RIN4 enhances its association with EXO70 to inhibit secretion of defense compounds.
Next steps:
AvrRpm1 also modifies at least 14 other NOI-domain containing proteins in Arabidopsis. Because AvrRpm1 enhances virulence in mutant plants lacking RIN4, some of these other proteins likely contribute to immune responses. Future work will address how these NOI-domain proteins contribute to defense, and how ADP-ribosylation blocks their function.
Thomas J. Redditt, Eui-Hwan Chung, Hana Zand Karimi, Natalie Rodibaugh, Yixiang Zhang, Jonathan C. Trinidad, Jin Hee Kim, Qian Zhou, Mingzhe Shen, Jeffery L. Dangl, David Mackey, Roger W. Innes (2019) AvrRpm1 Functions as an ADP-Ribosyl Transferase to Modify NOI Domain-Containing Proteins, Including Arabidopsis and Soybean RPM1-Interacting Protein4 https://doi.org/10.1105/tpc.19.00020