Apical Membrane Traffic In Pollen Tube Tips
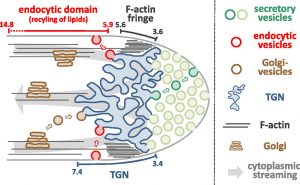 Pollen tubes elongate very rapidly at rates of several micrometer per minute and strictly in one direction: this process involves the intense local secretion of cell wall material at the tube tip. Consequently, there is a massive incorporation into the plasma membrane (PM) of excess membrane material that needs to be recycled endocytotically. Pollen tubes are extremely polarized: at the very tip, there is a strong accumulation of vesicles containing cell wall material; proximal to this region are all the other cell organelles, including a trans-Golgi network (TGN) and a subapical cortical F-actin fringe that is essential for tip growth. A key aim of a study by Grebnev et al. (10.1104/pp.20.00380) was to quantitatively determine the sites of bulk secretion and endocytic membrane recycling required for apical cell wall biogenesis at the tip of growing tobacco (Nicotiana tabacum) pollen tubes. The authors employed fluorescent markers, including the lipid dye FM4-64 as well as various enhanced yellow fluorescent protein (eYFP)-tagged transmembrane proteins, to study the steady-state distributions and dynamic behavior of these markers in elongating pollen tubes growing normally or treated with Brefeldin A (an inhibitor of secretion). The authors also modeled the patterns observed mathematically. Together, the experimental and theoretical data generated reveal quantitatively the sites of bulk secretion and endocytosis within apical and subapical PM regions at the tip of tobacco pollen tubes, respectively. The research presented also establishes the exact subapical positions of the cortical F-actin fringe and the trans-Golgi network relative to these sites. Finally, the authors demonstrate that within the identified subapical region of bulk endocytosis, constitutive recycling of membrane lipids occurs, which generally excludes the transmembrane proteins and appears to depend on the subapical trans-Golgi network but not the F-actin fringe.
Pollen tubes elongate very rapidly at rates of several micrometer per minute and strictly in one direction: this process involves the intense local secretion of cell wall material at the tube tip. Consequently, there is a massive incorporation into the plasma membrane (PM) of excess membrane material that needs to be recycled endocytotically. Pollen tubes are extremely polarized: at the very tip, there is a strong accumulation of vesicles containing cell wall material; proximal to this region are all the other cell organelles, including a trans-Golgi network (TGN) and a subapical cortical F-actin fringe that is essential for tip growth. A key aim of a study by Grebnev et al. (10.1104/pp.20.00380) was to quantitatively determine the sites of bulk secretion and endocytic membrane recycling required for apical cell wall biogenesis at the tip of growing tobacco (Nicotiana tabacum) pollen tubes. The authors employed fluorescent markers, including the lipid dye FM4-64 as well as various enhanced yellow fluorescent protein (eYFP)-tagged transmembrane proteins, to study the steady-state distributions and dynamic behavior of these markers in elongating pollen tubes growing normally or treated with Brefeldin A (an inhibitor of secretion). The authors also modeled the patterns observed mathematically. Together, the experimental and theoretical data generated reveal quantitatively the sites of bulk secretion and endocytosis within apical and subapical PM regions at the tip of tobacco pollen tubes, respectively. The research presented also establishes the exact subapical positions of the cortical F-actin fringe and the trans-Golgi network relative to these sites. Finally, the authors demonstrate that within the identified subapical region of bulk endocytosis, constitutive recycling of membrane lipids occurs, which generally excludes the transmembrane proteins and appears to depend on the subapical trans-Golgi network but not the F-actin fringe.