An opportunistic plant pathogen disrupts leaf microbiome through secretion of plant cell wall degrading enzymes
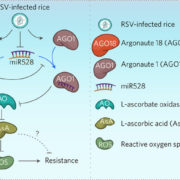
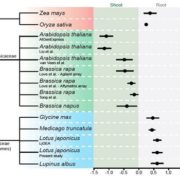
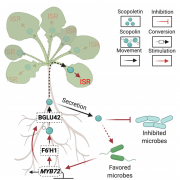
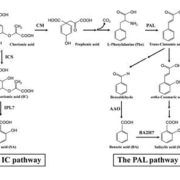
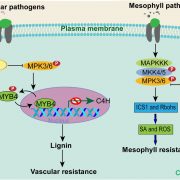
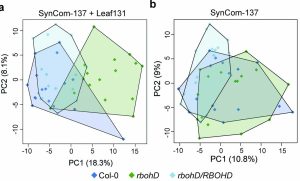 Healthy plants possess a functional immune system, and their leaves harbor a structured microbial community, including opportunistic pathogens. These opportunistic pathogens can trigger plant diseases under conducive conditions, such as when plant immunity is compromised, and the microbial community is disrupted. It remains elusive whether and how an opportunistic pathogen can directly disrupt the microbiome to cause disease. By using an immunocompromised Arabidopsis genotype, its complementation line, and a leaf-derived bacterial synthetic community (SynCom), Pfeilmeier et al. established a direct causative role of an opportunistic pathogen (Xanthomonas Leaf 131) in perturbing leaf microbial communities for disease development. By adopting a quantitative assay in Arabidopsis leaves, the authors confirmed leaf tissue degradation as a potential mode of virulence for this pathogenic strain. Further, they generated targeted deletion and mutated Xanthomonas Leaf 131 strains to provide both in vitro and in planta evidence that T2SS (type II secretions systems) secreted plant cell-wall degrading enzymes cause this characteristic leaf tissue degradation and favor specific commensal bacteria to perturb the leaf microbiome. This study revealed a mechanism of context dependent transition from commensalism to pathogenic behavior of an opportunistic pathogen by direct perturbation of the leaf microbiome. (Summary by Arijit Mukherjee ArijitM61745830) Nat. Microbiol. 10.1038/s41564-023-01555-z
Healthy plants possess a functional immune system, and their leaves harbor a structured microbial community, including opportunistic pathogens. These opportunistic pathogens can trigger plant diseases under conducive conditions, such as when plant immunity is compromised, and the microbial community is disrupted. It remains elusive whether and how an opportunistic pathogen can directly disrupt the microbiome to cause disease. By using an immunocompromised Arabidopsis genotype, its complementation line, and a leaf-derived bacterial synthetic community (SynCom), Pfeilmeier et al. established a direct causative role of an opportunistic pathogen (Xanthomonas Leaf 131) in perturbing leaf microbial communities for disease development. By adopting a quantitative assay in Arabidopsis leaves, the authors confirmed leaf tissue degradation as a potential mode of virulence for this pathogenic strain. Further, they generated targeted deletion and mutated Xanthomonas Leaf 131 strains to provide both in vitro and in planta evidence that T2SS (type II secretions systems) secreted plant cell-wall degrading enzymes cause this characteristic leaf tissue degradation and favor specific commensal bacteria to perturb the leaf microbiome. This study revealed a mechanism of context dependent transition from commensalism to pathogenic behavior of an opportunistic pathogen by direct perturbation of the leaf microbiome. (Summary by Arijit Mukherjee ArijitM61745830) Nat. Microbiol. 10.1038/s41564-023-01555-z