A well-oiled machine: two fatty-acid exporters involved in seed oil biosynthesis
A well-oiled machine: two fatty-acid exporters involved in seed oil biosynthesis
Mehran Dastmalchi
Oilseed plants such as canola (Brassica spp.) are a massive, multi-billion dollar industry, with their lipids contributing to food, cosmetics, polymers and biofuels. For the plant, seed oil is a form of high-density energy storage, much more efficient than carbohydrates. The oil is primarily composed of triacylglycerols (TAGs), which can contribute up to 50% of seed dry weight in plants such as Arabidopsis thaliana, or its commercially significant relative, canola. Structurally, TAGs are tri-esters consisting of a glycerol bound to three fatty acids.
 In the current issue of Plant Physiology, Li et al. (2020) further our understanding of fatty acid biosynthesis and transport in Arabidopsis seeds at a subcellular level. Fatty acids in plant seeds are synthesized de novo in plastids, acylated in the cytosol, and assembled into TAGs in the endoplasmic reticulum (ER). Consequently, there is a need for a plastid exporter of fatty acids and an ER importer of acyl-CoAs. The latter has been identified as ATP-BINDING CASSETTE TRANSPORTER 9 (ABCA9) and is localized to the ER membrane of seed cells (Kim et al., 2013). However, a seed-specific transporter, shuttling free fatty acids into the cytosol, has not been characterized. Here, the authors report two members of the Arabidopsis FATTY ACID EXPORT (FAX) protein family, FAX2 and FAX4, that are involved in the transport of fatty acids for TAG biosynthesis during seed development.
In the current issue of Plant Physiology, Li et al. (2020) further our understanding of fatty acid biosynthesis and transport in Arabidopsis seeds at a subcellular level. Fatty acids in plant seeds are synthesized de novo in plastids, acylated in the cytosol, and assembled into TAGs in the endoplasmic reticulum (ER). Consequently, there is a need for a plastid exporter of fatty acids and an ER importer of acyl-CoAs. The latter has been identified as ATP-BINDING CASSETTE TRANSPORTER 9 (ABCA9) and is localized to the ER membrane of seed cells (Kim et al., 2013). However, a seed-specific transporter, shuttling free fatty acids into the cytosol, has not been characterized. Here, the authors report two members of the Arabidopsis FATTY ACID EXPORT (FAX) protein family, FAX2 and FAX4, that are involved in the transport of fatty acids for TAG biosynthesis during seed development.
Another family member, FAX1, is also an exporter of fatty acids and is localized to the plastid inner envelope (Li et al., 2015). Unlike the newly characterized members, FAX1 primarily functions in the leaf and flower, two alternative but minor tissues for TAG production. Interestingly, when AtFAX1 was overexpressed under the control of a seed-specific promoter, plants yielded 21–33% more oil per seed compared to wild-type (WT) Arabidopsis (Tian et al., 2018). The current study characterizes FAX2 and FAX4, which specifically mediate plastid fatty acid export in seeds during the seed-filling stage.
The authors identified seven genes predicted to encode FAX transporters in Arabidopsis. FAX2 and FAX4 belonged to the same phylogenetic clade as previously characterized FAX1. AtFAX2 and AtFAX4 are highly expressed during the early stages of seed development in the embryos. At a subcellular level, FAX2 and FAX4 were localized to the chloroplast membrane; however, the two proteins do not appear to interact molecularly. Therefore, their respective export functions are most likely carried out independently.
T-DNA insertion mutations for both genes were analyzed separately (fax2, fax4) and together (fax2fax4), with the double mutation rendering the most distinct phenotypes, suggesting functional redundancy. Although the relative fatty acid composition in fax2fax4 mutants was unaltered compared to wild type, the overall lipid content was reduced. As a result, a larger percentage of seeds were defective (shrunken or small), as seed-filling is dependent on storage lipid production.
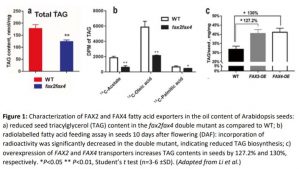
An in-depth analysis of TAG biosynthesis in the mutants revealed that a) plastid lipid content was increased in the mutants, and b) total TAG content was reduced (approximately 30% less than wild type) (Figure 1a). Blocked lipid export in fax2fax4 seeds led to a buildup of fatty acids in the plastid and presumably reduced substrate availability for TAG biosynthesis in the ER. However, the relative composition of TAG molecular species was not affected by FAX2 and FAX4, suggesting that they are broad-range transporters of fatty acids.
To dissect the substrate specificity of the two transporters, the researchers conducted an elegant feeding study using radioactive carbon: (14C)-acetate, -oleic acid, and -palmitic acid. Developing seeds, ten days after flowering (DAF), were fed radiolabelled substrates, and their incorporation into downstream TAG fractions was measured in disintegrations per minute (DPM) (Figure 1b). The incorporation of radioactive fatty acid content, when fed 14C-acetate and 14C-oleic acid, was drastically reduced in the double mutant as compared to WT. By contrast, when seeds were fed 14C-palmitic acid, the radioactive content was only marginally reduced. The feeding study indicated that FAX2 and FAX4 are chloroplast fatty-acid transporters, with substrate selectivity. Exploring if the two transporters are identical in substrate range, as is suggested in the present work, could benefit from a broader array of radiolabelled fatty acids or a heterologous transport assay.
Ultimately, the translational importance of characterizing lipid transport lies in boosting seed oil yield in crops such as canola. As with previous studies on fatty acid transporters, including FAX1 and ABCA9 (Kim et al., 2013; Tian et al., 2018), overexpression lines of FAX2 and FAX4 improved seed lipid content (Figure 1c). Transporters could be deployed synergistically to potentially improve both plastidic export (via FAX) and ER import (via ABCA9). Furthermore, efforts can be made in coupling improved substrate availability, with higher rates of acylation and TAG biosynthesis, through enzyme engineering. Targets for such work include diacylglycerol acyltransferase, which has separately been exploited to improve seed oil content (Katavic et al., 1995; Jako et al., 2001).
In the long run, field trials of mutant lines with enhanced seed lipid transport and metabolism can determine the viability of such an approach to improving oil content. Any gains in oil yield must be weighed against off-target effects, such as pollen wall composition and cuticular waxes, which are also derived from exported fatty acids, similar to TAGs (Bates et al., 2013). The current study by Li et al. has furthered our knowledge of intracellular fatty acid biosynthesis and transport, demonstrating its importance for seed TAG content. Further unraveling of the physiological impact of FAX transporters and the nuances of each isoform can promote future seed oil research.
REFERENCES
Bates PD, Stymne S, Ohlrogge J (2013) Biochemical pathways in seed oil synthesis. Curr Opin Plant Biol. doi: 10.1016/j.pbi.2013.02.015
Jako C, Kumar A, Wei Y, Zou J, Barton DL, Giblin EM, Covello PS, Taylor DC (2001) Seed-specific over-expression of an arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol. doi: 10.1104/pp.126.2.861
Katavic V, Reed DW, Taylor DC, Giblin EM, Barton DL, Zou J, MacKenzie SL, Covello PS, Kunst L (1995) Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol. doi: 10.1104/pp.108.1.399
Kim S, Yamaoka Y, Ono H, Kim H, Shim D, Maeshima M, Martinoia E, Cahoon EB, Nishid I, Lee Y (2013) AtABCA9 transporter supplies fatty acids for lipid synthesis to the endoplasmic reticulum. Proc Natl Acad Sci U S A 110: 773–778
Li N, Gügel IL, Giavalisco P, Zeisler V, Schreiber L, Soll J, Philippar K (2015) FAX1, a Novel Membrane Protein Mediating Plastid Fatty Acid Export. PLOS Biol 13: e1002053
Li N, Meng H, Li S, Zhang Z, Zhao X, Shufeng W, Liu A, Li Q, Song Q, Li X, Guo L, Li H (2020) Two plastid fatty acid exporters contribute to seed oil accumulation in Arabidopsis. Plant Phys 182: https://doi.org/10.1104/pp.19.01344
Tian Y, Lv X, Xie G, Zhang J, Xu Y, Chen F (2018) Seed-specific overexpression of AtFAX1 increases seed oil content in Arabidopsis. Biochem Biophys Res Commun 500: 370–375