A Tonoplast Calcineurin B-Like Protein and Stomatal Movement
SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) comprise a highly conserved superfamily of proteins in all eukaryotic cells and play important roles in membrane fusion events involved in the delivery of membranes, proteins, and soluble cargos. SNARES form a core complex to bring vesicle and target membrane surfaces together, thereby driving secretion as well as the traffic of vesicles between endosomal compartments. Beyond their canonical role in membrane fusion, a few SNAREs are also known to interact with 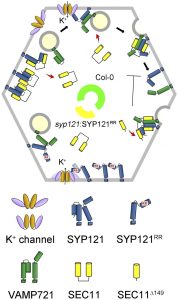 ion channels and affect their regulation. The plasma membrane SNARE SYP121 of Arabidopsis is the best-known example. More specifically, SYP121 interacts with the K+ channels KC1 and KAT1, altering channel gating to promote K+ uptake. Channel binding is specific for SYP121: it depends on a conserved N-terminal motif defined by the sequence F9xRF within SYP121. Much of vesicle traffic at the Arabidopsis plasma membrane, however, is subject to the protein SEC11, which also selectively binds with SYP121. How the binding of SEC11 with SYP121 is coordinated with SYP121 interactions with K+ channels is poorly understood, as both SEC11 and the channels are thought to compete for the same SNARE binding site. Zhang et al. (10.1104/pp.18.01315) now identify a second binding motif within the N-terminus of SYP121 and demonstrate that this motif affects SEC11 binding independently of the F9xRF motif that is shared with the K+ channels. This second, previously unrecognized motif is centered on residues R20R21 of SYP121 and is essential for SEC11 interaction with SYP121. Mutation of the R20R21 motif blocked vesicle traffic without
ion channels and affect their regulation. The plasma membrane SNARE SYP121 of Arabidopsis is the best-known example. More specifically, SYP121 interacts with the K+ channels KC1 and KAT1, altering channel gating to promote K+ uptake. Channel binding is specific for SYP121: it depends on a conserved N-terminal motif defined by the sequence F9xRF within SYP121. Much of vesicle traffic at the Arabidopsis plasma membrane, however, is subject to the protein SEC11, which also selectively binds with SYP121. How the binding of SEC11 with SYP121 is coordinated with SYP121 interactions with K+ channels is poorly understood, as both SEC11 and the channels are thought to compete for the same SNARE binding site. Zhang et al. (10.1104/pp.18.01315) now identify a second binding motif within the N-terminus of SYP121 and demonstrate that this motif affects SEC11 binding independently of the F9xRF motif that is shared with the K+ channels. This second, previously unrecognized motif is centered on residues R20R21 of SYP121 and is essential for SEC11 interaction with SYP121. Mutation of the R20R21 motif blocked vesicle traffic without
uncoupling the effects of SYP121 on solute and K+ uptake associated with the F9xRF motif.



