A novel Trojan horse for molecule delivery into plants
Marc-Sven Roell1
ORCID ID: 0000-0003-2714-8729
1Institute of Plant Biochemistry, Heinrich-Heine University Düsseldorf, Universitätsstrasse 1, 40225 Düsseldorf, Germany
The agronomic application of nanotechnology harbors huge potential for future agriculture (Landry and Mitter, 2019). Within the last years, nanocarriers have emerged as vehicles for the delivery of cargo (RNA, DNA, protein, plant protection substances) into the plant cell (Wang et al., 2016). RNA-induced gene silencing (also known as RNA interference) is a reliable method to study and alter genetic form and function of plants. Nanocarriers offer the possibility to directly deliver small interfering RNA (double stranded RNA of 20-25 base pairs, siRNA) into the plant cell without involving a biological carrier (e.g., viruses) or genetic transformation (Cunningham et al., 2018).
Carbon-based nanostructures such as carbon dots and single-walled carbon nanotubes (Demirer et al., 2019; Demirer et al., 2020) are valuable alternatives to common transformation methods, since they do not require genetic transformation (Wang et al., 2016) and avoid heavy metal nanoparticles, usually used for biolistic transformation (Klein et al., 1987). Although, single-walled carbon nanotubes and carbon dots share most beneficial properties, they differ in size. Single walled carbon nanotubes are ~1 nm in diameter and up 1000 nm in length and, in contrast, carbon dots are on average ~3 nm in size (Demirer et al., 2020; Schwartz et al., 2020). Benefits of carbon-based nanostructures are a high aspect ratio (i.e. ratio of length to width), good biocompatibility (especially compared with metal nanoparticles), and the ability to protect bound biomolecules from cellular metabolism and degradation (Demirer et al., 2019; Kwak et al., 2019). Furthermore, tissue-specific tracking based on their fluorescent properties and intracellular ‘on demand’ cargo release holds great promise for broad application (Wang et al., 2016; Cunningham et al., 2018). The cellular uptake of nanocomplexes is facilitated by endocytosis (Figure 1) and intracellular release of the nanocomplex is caused by osmotically driven endosome burst, called the ‘proton-sponge’ effect (Behr, 1997). The utilization of nanoparticles that respond either to intracellular stimuli (pH, redox state, enzymes) or external stimuli (light or ultrasound) facilitates controlled cargo release within the cell (Wang et al., 2016; Cunningham et al., 2018).
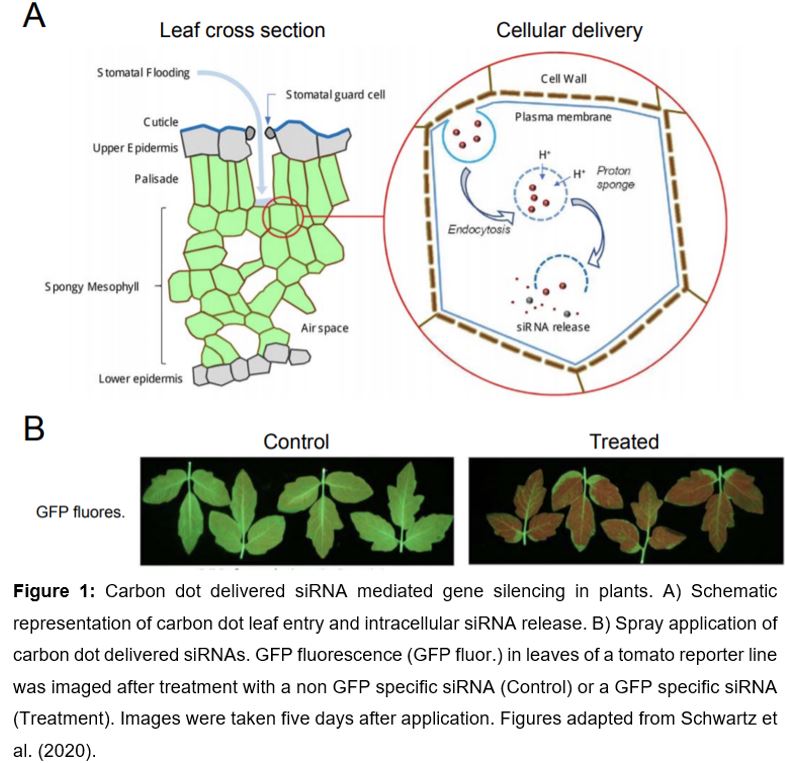 In this issue of Plant Physiology, Schwartz et al. (2020) establish the use of carbon dots for delivery of siRNAs as a novel tool for gene silencing in plants. Simple spray application of carbon dots results in highly efficient reporter and endogenous gene silencing in model and crop species and holds great potential for field application. In their study, Schwartz et al. (2020) optimized the chemical synthesis and purification of carbon dots before application. The low-cost ‘bottom up’ synthesis of carbon dots is based on a one-pot reaction using citrate or glucose with branched polyethyleneimine for carbon dot surface functionalization. Size-exclusion chromatography of prepared carbon dots revealed that carbon dots with an average size of 3.8 nm in diameter, combined with 22mer siRNAs are most efficient for in planta gene silencing.
In this issue of Plant Physiology, Schwartz et al. (2020) establish the use of carbon dots for delivery of siRNAs as a novel tool for gene silencing in plants. Simple spray application of carbon dots results in highly efficient reporter and endogenous gene silencing in model and crop species and holds great potential for field application. In their study, Schwartz et al. (2020) optimized the chemical synthesis and purification of carbon dots before application. The low-cost ‘bottom up’ synthesis of carbon dots is based on a one-pot reaction using citrate or glucose with branched polyethyleneimine for carbon dot surface functionalization. Size-exclusion chromatography of prepared carbon dots revealed that carbon dots with an average size of 3.8 nm in diameter, combined with 22mer siRNAs are most efficient for in planta gene silencing.
Whereas previous nanoparticle approaches in plants rely on particle bombardment (Klein et al., 1987) or leaf-infiltration (Demirer et al., 2019; Demirer et al., 2020), carbon dots with siRNA cargo are efficient for gene silencing upon low-pressure spraying application (Schwartz et al., 2020). Green fluorescent protein (GFP) transcript- and protein abundance were more than 80 % reduced in reporter lines of wild tobacco (Nicotiana benthamiana) and tomato (Solanum lycopersicum) five days after application (Figure 1). Remarkably, systemic spreading of silencing was observed in emerging leaves, twelve days after application due to intercellular and long-distance movement of siRNAs (Melnyk et al., 2011).
Reporter gene independent validation was achieved by targeting the endogenous H and I subunit of magnesium chelatase. Magnesium chelatase catalyzes the insertion of magnesium into protoporphyrin IX, an essential step in chlorophyll biosynthesis and knockdown results in leave bleaching. Comparable to GFP silencing, endogenous gene silencing also reached an 80 % reduction in mRNA level.
Carbon dot-based siRNA delivery into plant cells expands the spectrum of carbon-based nanoparticles for molecule delivery into plant cells and is an exciting tool for fundamental and applied plant science. The simple spray application makes it well suited for large-scale agricultural use. Further, prepared carbon dots are also persistent and remain overall efficacy for at least one-week storage before application. Nevertheless, a comparable high efficacy with less established plant species needs to be shown and fine-tuning cellular entry and intracellular siRNA release can further optimize broad spectrum application.
Literature cited
Behr JP (1997) The proton sponge: A trick to enter cells the viruses did not exploit. Chimia 51: 34–36
Cunningham FJ, Goh NS, Demirer GS, Matos JL, Landry MP (2018) Nanoparticle-Mediated Delivery towards Advancing Plant Genetic Engineering. Trends Biotechnol 36: 882–897
Demirer GS, Zhang H, Goh NS, Pinals RL, Chang R, Landry MP (2020) Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci Adv 6: eaaz0495
Demirer GS, Zhang H, Matos JL, Goh NS, Cunningham FJ, Sung Y, Chang R, Aditham AJ, Chio L, Cho M-J, et al (2019) High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nature Nanotechnology 14: 456–464
Klein TM, Wolf ED, Wu R, Sanford JC (1987) High-velocity microprojectiles for delivering nucleic acids into living cells. Nature 327: 70–73
Kwak S-Y, Lew TTS, Sweeney CJ, Koman VB, Wong MH, Bohmert-Tatarev K, Snell KD, Seo JS, Chua N-H, Strano MS (2019) Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nature Nanotechnology 14: 447–455
Landry MP, Mitter N (2019) How nanocarriers delivering cargos in plants can change the GMO landscape. Nature Nanotechnology 14: 512–514
Melnyk CW, Molnar A, Baulcombe DC (2011) Intercellular and systemic movement of RNA silencing signals. EMBO J 30: 3553–3563
Wang P, Lombi E, Zhao F-J, Kopittke PM (2016) Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci 21: 699–712