A New Plant Component of the Conserved Machinery for Cellular Trafficking
Garcia et al. discover a plant-specific component of TRAPP membrane trafficking complexes with important roles in plant development. Plant Cell https://doi.org/10.1105/tpc.20.00044.
By Veder Garcia and Zhi-Yong Wang
Department of Plant Biology, Carnegie Institution for Science, Stanford, CA
Background: Cells use membrane vesicles to deliver proteins to their target cellular locations. In yeast and animals, Transport Protein Particle (TRAPP) complexes act as linkers that tether these membrane vesicles to the membranes of their target compartments. Plant genomes encode proteins similar to the proteins in yeast and animal TRAPP complexes. Mutations in some of these subunits in Arabidopsis thaliana disrupt vesicle trafficking, cell division, and plant development. However, the TRAPP complexes in plants have not yet been fully characterized at the molecular level.
Question: We wanted to know which proteins make up the TRAPP complexes in plants and whether new proteins have evolved to provide unique functions required for plant development. We used antibodies and mass spectrometry to identify all of the proteins of plant TRAPP complexes and performed genetic analyses to investigate their functions.
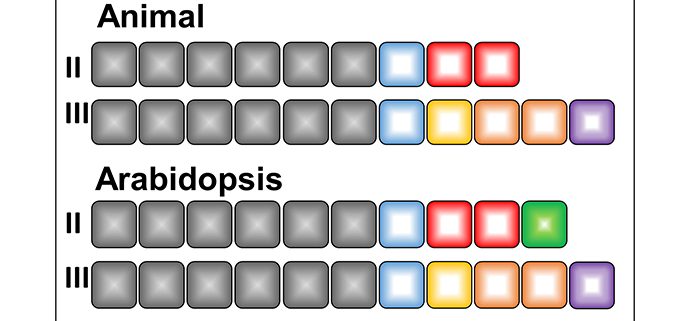
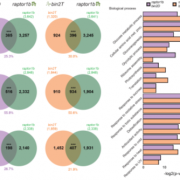
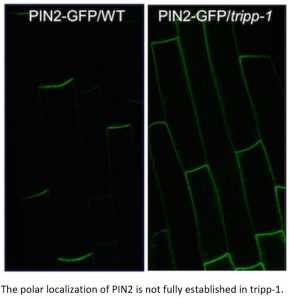 Findings: We identified 14 proteins in plant TRAPP complexes. These proteins include counterparts to all 13 animal TRAPP proteins, plus one protein, which we named TRAPP-Interacting Plant Protein (TRIPP), that is only encoded in the genomes of multicellular plants. We discovered that this protein is part of the TRAPPII complex but not TRAPPIII and that it is found in two specific cellular locations (the trans-Golgi network and cell plates of dividing cells). An Arabidopsis mutant lacking the TRIPP protein has several defects that suggest this protein plays important roles in cell plate formation, polar localization of a transporter of the hormone auxin, reproduction, and seedling development in the dark. We identified TRIPP as a plant-specific component of the otherwise highly conserved TRAPPII complex, with functions in unique vesicle trafficking processes in plant cells.
Findings: We identified 14 proteins in plant TRAPP complexes. These proteins include counterparts to all 13 animal TRAPP proteins, plus one protein, which we named TRAPP-Interacting Plant Protein (TRIPP), that is only encoded in the genomes of multicellular plants. We discovered that this protein is part of the TRAPPII complex but not TRAPPIII and that it is found in two specific cellular locations (the trans-Golgi network and cell plates of dividing cells). An Arabidopsis mutant lacking the TRIPP protein has several defects that suggest this protein plays important roles in cell plate formation, polar localization of a transporter of the hormone auxin, reproduction, and seedling development in the dark. We identified TRIPP as a plant-specific component of the otherwise highly conserved TRAPPII complex, with functions in unique vesicle trafficking processes in plant cells.
Next steps: We still don’t know exactly how TRIPP functions at the molecular level, and we would like to test several hypotheses. TRIPP might help TRAPPII bind with other proteins, or it might help stabilize the TRAPPII complex to control the ratio between the TRAPPII and TRAPPIII complexes, which share the same core components. Exploring the structure of the plant TRAPPII complex and TRIPP will also be important for understanding their functions.