A membrane-bound cellulase in time and space
Nagashima et al. investigate molecular mechanisms for the sorting of a membrane protein KORRIGAN 1 (KOR1) in plant cells.
Plant Cell https://doi.org/10.1105/tpc.19.00714
By Y. Nagashima1, and H. Koiwa1,2
1Vegetable and Fruit Improvement Center and Department of Horticultural Sciences, Texas A&M University
2Molecular and Environmental Plant Sciences, Texas A&M University
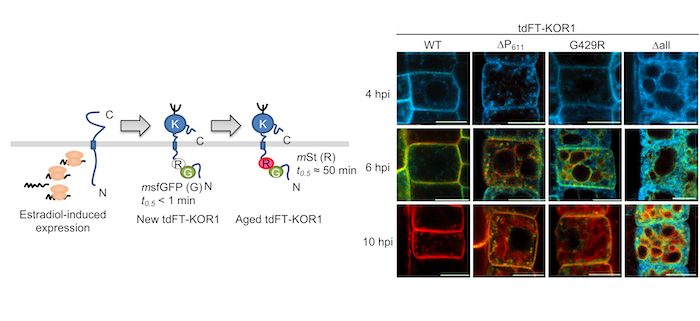
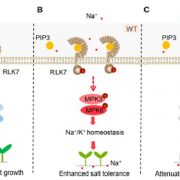
Background: KORRIGAN1 (KOR1) is a membrane-bound cellulase involved in cellulose synthesis in plants. KOR1 is a link connecting protein N-glycosylation, a major post-translational modification, with plant growth and tolerance to abiotic stresses like salt stress. In Arabidopsis, KOR1 cycles between the plasma membrane (PM) and trans-Golgi network (TGN) and is targeted from the TGN to the cell plate during cell division in the root tip. Genetic mutations in KOR1 promote the accumulation of KOR1 protein at the tonoplast (vacuolar membrane), likely for degradation.
Question: KOR1 shows dynamic changes in its localization in cells; however, the delivery route of KOR1 to multiple organelles was unclear. Which path does KOR1 go through to various organelles? Which part of the KOR1 protein is important for its transport? How is KOR1 transport influence stress tolerance? These are questions we aimed to address.
Findings: To understand the delivery route of KOR1, we used a tandem fluorescent timer (tdFT) technology to analyze the location and age of KOR1 simultaneously. Compromised KOR1 protein, likely mimicking damaged/expired KOR1, accumulated in the tonoplast through the PM. We established that a dileucine motif on KOR1 was necessary for KOR1 to move from the plasma membrane to the tonoplast, because KOR1 with a mutation in the dileucine motif was locked at the PM. We also determined that KOR1 temporarily moved from the PM to the TGN under salt stress; however, KOR1 with a mutation in the dileucine motif was locked at the PM and inhibited root growth under salt stress. These findings revealed that proper localization of KOR1 is necessary for plant salt acclimation.
Next steps: Here we showed the compromised KOR1 was delivered to the tonoplast. However, the cellular mechanism that recognizes and delivers compromised KOR1 to the tonoplast remains unclear. We would like to identify the factors that sense the damaged/expired KOR1 at PM/TGN. Furthermore, we plan to optimize the tdFT system for further applications in plants.
Yukihiro Nagashima, Zeyang Ma, Xueting Liu, Xiaoning Qian, Xiuren Zhang, Antje von Schaewen, Hisashi Koiwa (2020). Multiple quality control mechanisms in the ER and TGN determine subcellular dynamics and salt-stress tolerance function of KORRIGAN 1. Plant Cell; DOI: https://doi.org/10.1105/tpc.19.00714.