A cross-kingdom conserved ER-phagy receptor maintains ER homeostasis during stress (eLIFE)
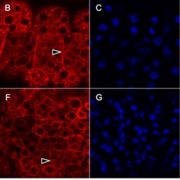
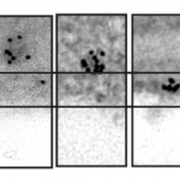
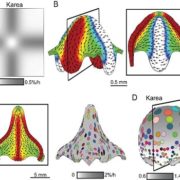
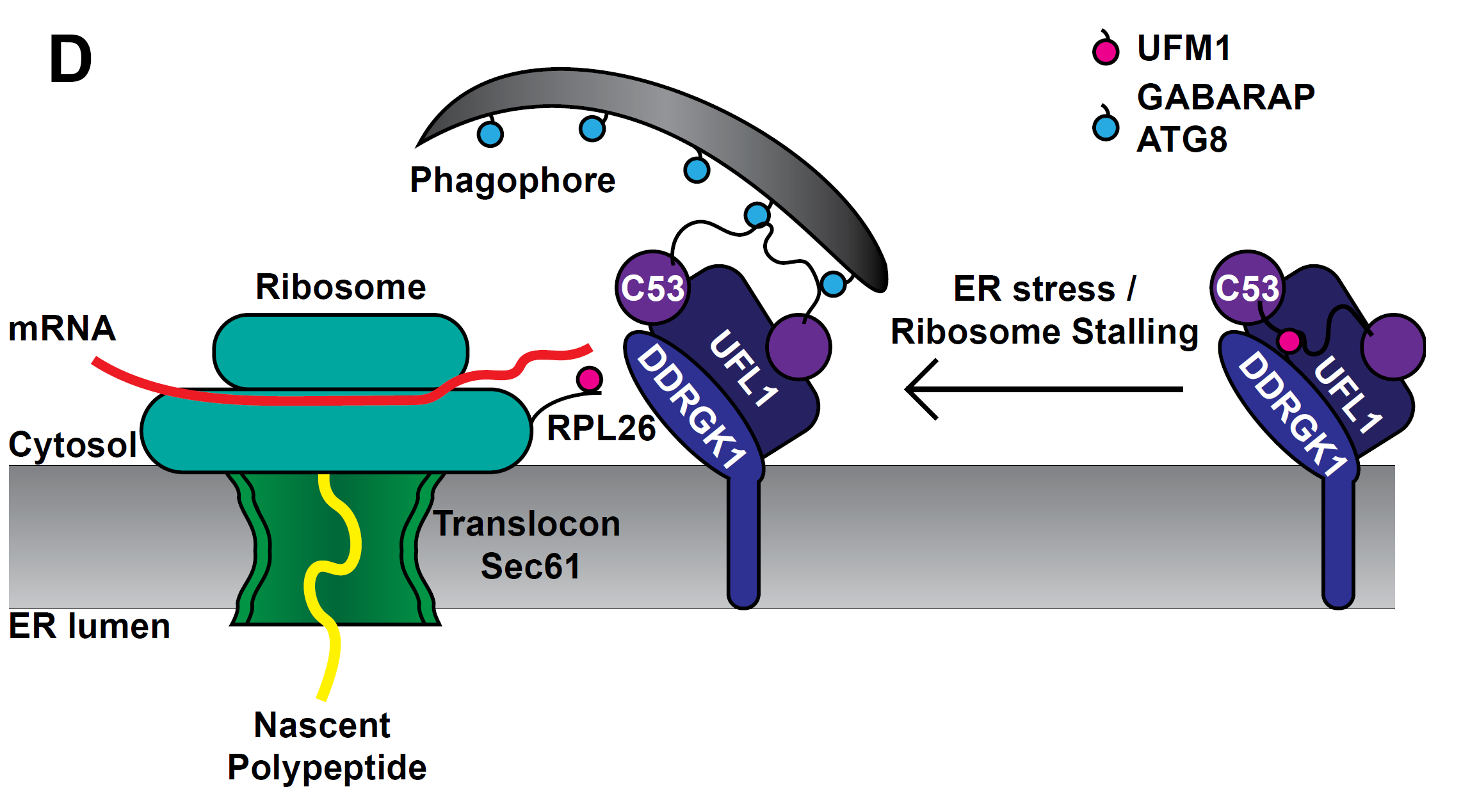 Quality control of the protein folding mechanism in the endoplasmic reticulum (ER), which selectively eliminates or recycles unwanted cytoplasmic components, is recognized by specific autophagy receptors. Selective removal of certain ER domains by the autophagy pathway (termed as ER-phagy) is controlled by these receptors that recruit specific cargo through interaction with the Autophagy-related protein 8 (ATG8) and then delivered for degradation and recycling. Stephani et al. identified a new receptor, C53, which is conserved across kingdoms that is specifically recruited into autophagosomes during ER stress. C53 binds ATG8 in A. thaliana, Marchantia polymorpha and human HeLa cells, and C53 localizes to the transport vesicles during ER stress. C53-ATG8 interaction is mediated via C53 conserved intrinsically disorder region with a novel consensus motif “IDWG” which is located at the highly flexible region. Interestingly, this C53 is neither activated by association with unfolded protein response sensors or ER-associated degradation pathway substrates, nor with the signal recognition particle. C53 is only activated upon ribosome stalling during co-translational protein translocation. The C53 mutant in plants is highly sensitive to ER stress caused by phosphate starvation. Overall, this study sheds light on a unique ER-recycling receptor that maintains cellular quality control. (Summary by Min May Wong @wongminmay) eLife 10.7554/elife.58396
Quality control of the protein folding mechanism in the endoplasmic reticulum (ER), which selectively eliminates or recycles unwanted cytoplasmic components, is recognized by specific autophagy receptors. Selective removal of certain ER domains by the autophagy pathway (termed as ER-phagy) is controlled by these receptors that recruit specific cargo through interaction with the Autophagy-related protein 8 (ATG8) and then delivered for degradation and recycling. Stephani et al. identified a new receptor, C53, which is conserved across kingdoms that is specifically recruited into autophagosomes during ER stress. C53 binds ATG8 in A. thaliana, Marchantia polymorpha and human HeLa cells, and C53 localizes to the transport vesicles during ER stress. C53-ATG8 interaction is mediated via C53 conserved intrinsically disorder region with a novel consensus motif “IDWG” which is located at the highly flexible region. Interestingly, this C53 is neither activated by association with unfolded protein response sensors or ER-associated degradation pathway substrates, nor with the signal recognition particle. C53 is only activated upon ribosome stalling during co-translational protein translocation. The C53 mutant in plants is highly sensitive to ER stress caused by phosphate starvation. Overall, this study sheds light on a unique ER-recycling receptor that maintains cellular quality control. (Summary by Min May Wong @wongminmay) eLife 10.7554/elife.58396