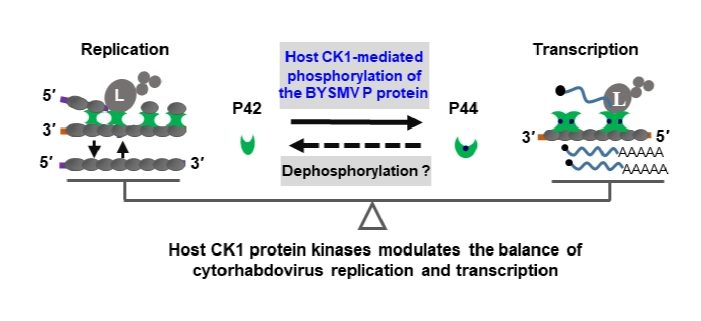
A Balancing act between virus replication and transcription
Gao et al. demonstrate that CK1 regulates plant cytorhabdovirus infections in both plants and insects. Plant Cell https://doi.org/10.1105/tpc.20.00369
By Qiang Gao and Xian-Bing Wang, China Agricultural University
Background: Phosphorylation is a widespread post-translational modification of cellular proteins in eukaryotic cells. For instance, Casein kinase 1 (CK1) enzymes are highly conserved Ser/Thr protein kinases that regulate critical aspects of development in yeast, plants, and animals. Accumulating evidence suggests that many host protein kinases play pivotal roles when plants are infected by a virus by phosphorylating viral proteins. Rhabdoviruses are a group of viruses with negative-sense single-stranded RNA genomes that cause significant losses to agriculture production worldwide when they infect crops. Most plant rhabdoviruses are obligately transmitted by arthropod vectors in a persistent propagative manner. Recent breakthroughs in reverse genetics systems applied to plant rhabdoviruses now allow an investigation of virus-insect-plant interactions.
Question: We wanted to know whether a conserved protein kinase might be implicated in the cross-kingdom infections of host plants and insect vectors by plant rhabdoviruses. How does protein kinases regulate infections of plant rhabdoviruses?
Findings: We found that the phosphoprotein (P) of barley yellow striate mosaic virus (BYSMV), a plant cytorhabdovirus, was phosphorylated by plant and insect CK1 protein kinases. Mass spectrometry analysis revealed that CK1 phosphorylated a Ser-rich (SR) motif at the C terminal intrinsically disordered region of BYSMV P. Phosphorylation of BYSMV P results in the production of two P forms with different mobilities corresponding to 42 kDa (P42) and 44 kDa (P44) in protein gels. Using a reverse genetics system in BYSMV, we found that BYSMV P42 and P44 stimulated viral replication and transcription, respectively. Overexpression of CK1, or a dominant-negative CK1 mutant, impaired the balance between P42 and P44, thereby compromising viral infection. Our results revealed that BYSMV recruits conserved CK1 kinases for cross-kingdom infections in host plants and insect vectors.
Next steps: We wish to determine the spatial and temporal expression of the different phosphorylation forms of BYSMV P, as well as their functions during BYSMV infections in host plants and insect vectors. Whether CK1 protein kinases are common modulators of other plant and animal rhabdoviruses also remains an open question for investigation.
Gao et al. (2020). Casein kinase 1 regulates cytorhabdovirus replication and transcription by phosphorylating a phosphoprotein serine-rich motif Plant Cell https://doi.org/10.1105/tpc.20.00369