XOAT1- At the heart of plant polysaccharide acetylation
Lunin et al. describe a structural and biochemical analysis of the enzyme XOAT1, responsible for the acetylation of the cell wall component xylan, with potential applications in cell wall modification for better biomass valorization. The Plant Cell (2020) https://doi.org/10.1105/tpc.20.00028
By Vivek Bharadwaj, Markus Alahuhta, Vladimir V. Lunin, Hsin-Tzu Wang
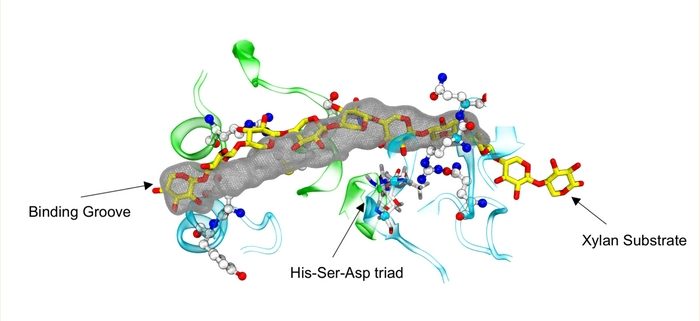
Background: Xylan is an integral part of hemicellulose, which is an essential component of the plant cell wall, playing a major role in its integral architectural framework and providing inherent mechanical strength. Xylose units in xylan are commonly decorated with sugar and acetyl functional groups. The enzyme xylan O-acetyl transferase 1 (XOAT1) is primarily responsible for catalyzing the addition of acetyl groups to the polymer backbone. From a biomass valorization perspective, the acetyl groups enable key interactions with other components of the cell wall, but they are also a major deterrent to the enzymatic digestion of plant biomass. Hence, knowledge of the mechanism employed by XOAT1 is key to understanding plant cell wall biosynthesis and developing new routes to engineer plants with improved or modified cell wall properties.
Question: We wanted to gain a molecular level understanding of the enzymatic mechanism of the acetylation process catalyzed by XOAT1. We sought to understand the structure of the enzyme at the atomic level, its underlying catalytic mechanism and the important amino acid residues responsible for substrate binding and catalysis. The catalytic mechanism and three-dimensional structure of XOAT1 were previously unknown.
Findings: In this study, we determined the atomic structure of XOAT1 using X-ray crystallography. We identified the active site located in a groove that can accommodate xylan oligomers and has a catalytically important Ser-His-Asp triad situated at its center. We demonstrated that the enzyme can use various acetyl donor molecules for the reaction and that the mechanism involves the formation of an acyl-enzyme intermediate. Based on insights from molecular simulations of substrate-bound XOAT1, we propose a detailed reaction mechanism that involves two stages: first transfer of an acetyl moiety to the Ser residue of the triad, followed by the subsequent transfer of the acetyl group from Ser to the xylan oligomer.
Next steps: Our study lays the foundations for engineering xylan acetylation patterns and content in plant biomass. These molecular level discoveries may now be used to design, engineer, and produce next-generation plants and structurally defined bio-polymers with custom properties.
Vladimir Lunin et al. (2020). Molecular mechanism of polysaccharide acetylation by the Arabidopsis xylan O-acetyltransferase XOAT1. Plant Cell DOI: https://doi.org/10.1105/tpc.20.00028