Threonine Phosphorylation Regulates Polar Localization of the Boric Acid Transporter NIP5;1 in Root Cells
IN BRIEF by Gregory Bertoni gbertoni@aspb.org
Proper localization of proteins in the plasma membrane is critical for proper functioning of plant cells, but the underlying mechanisms are poorly understood (Łangowski et al, 2016). This is especially true for transporter proteins that move necessary nutrients into and out of cells and organelles. For example, proper polar localization of PIN-FORMED auxin transporters in the rootward domain of the plasma membrane of root cells allows efficient auxin transport in the longitudinal direction (Feraru et al., 2011). Less is known about the mechanisms responsible for polar localization of proteins in the radial direction, the direction that absorbed nutrients must move in order to reach the stele for transport to aerial parts of the plant.
Boron is a minor, but essential element in plant nutrition that is important for cell wall structure and integrity. It is imported from the soil as boric acid by root epidermal cells and then exported to xylem tissue for transport to the rest of the plant. Although boric acid can slowly diffuse across membranes directly, under boron-limited conditions the boric acid channel NODULIN INTRINSIC PROTEIN 5;1 (NIP5;1) is required to allow sufficient boron uptake for normal growth (Takano et al., 2006). NIP5;1, a member of the aquaglyceroporin family of transport proteins, is preferentially localized in the plasma membrane on the soil side of root epidermal cells in Arabidopsis thaliana. Wang et al., (2017) use domain swapping, deletion analysis, and site-directed mutagenesis to investigate the mechanism underlying NIP5;1 polar localization, and they show that it depends upon phosphorylation of specific conserved threonine residues.
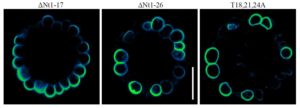 NIP5;1 contains three copies of a a distinctive Thr-Pro-Gly (TPG) repeat in its N terminus that is not found in closely related proteins that do not show polar localization. Deletion of 17 N-terminal residues (up to, but not including, the TPG repeat) had little effect on polar localization of GFP-NIP5;1, which displayed a wild-type localization pattern in root cells, being found primarily on the soil-facing side of cells (see figure, left panel). However, deletion of an additional nine residues (containing the triple TPG repeat) nearly eliminated polar localization (see figure, middle panel).
NIP5;1 contains three copies of a a distinctive Thr-Pro-Gly (TPG) repeat in its N terminus that is not found in closely related proteins that do not show polar localization. Deletion of 17 N-terminal residues (up to, but not including, the TPG repeat) had little effect on polar localization of GFP-NIP5;1, which displayed a wild-type localization pattern in root cells, being found primarily on the soil-facing side of cells (see figure, left panel). However, deletion of an additional nine residues (containing the triple TPG repeat) nearly eliminated polar localization (see figure, middle panel).
Whereas replacement of the three Thr residues in the TPG repeat with the phosphomimic Asp (T18,21,24D) had little effect on polar localization, replacement with nonphosphorylatable Ala residues (T18,21,24A) eliminated normal polar localization (see figure, right panel). In addition, the T18,21,24A mutant sequence was unable to complement the growth defect of nip5;1 in boron-limited conditions. This was not due to a defect in transport activity, as it was shown that the T18,21,24A mutant protein expressed in Xenopus laevis oocytes had wild-type boric acid transport activity.
Treatment with brefeldin A, which inhibits recycling of internalized plasma membrane proteins, caused formation of aggregates containing GFP-NIP5;1, but none were seen with the T18A.T21A.T24A mutant, suggesting that threonine phosphorylation in the TPG repeat accelerated the endocytosis of NIP5;1. In addition, polar localization was greatly diminished in ap2m mutants, which are deficient in adaptor protein 2, a core component required for clathrin-mediated endocytosis.
In summary, Wang et al. have shown that proper polar localization of the boric acid transporter NIP5;1 is maintained by threonine phosphorylation in the N-terminal TPG repeat and that proper localization mediated by clathrin-dependent endocytosis is necessary for proper growth of plants under boron-limited conditions. It will be interesting not only to further analyze the mechanism of polar localization of NIP5;1 but also to discover whether the NIP5;1 N-terminal TPG domain can function in chimeric proteins to direct the polar localization of other membrane proteins.
REFERENCES
Feraru, E., Feraru, M.I., Kleine-Vehn, J., Martinière, A., Mouille, G., Vanneste, S., Vernhettes, S., Runions, J., and Friml, J. (2011). PIN polarity maintenance by the cell wall in Arabidopsis. Curr. Biol. 21: 338–343.
Łangowski, Ł., Wabnik, K., Li, H., Vanneste, S., Naramoto, S., Tanaka, H., and Friml, J. (2016). Cellular mechanisms for cargo delivery and polarity maintenance at different polar domains in plant cells. Cell Discov. 2: 16018.
Takano, J., Wada, M., Ludewig, U., Schaaf, G., von Wirén, N., and Fujiwara, T. (2006). The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18: 1498–1509.
Wang, S., Yoshinari, A., Shimada, T., Hara-Nishimura, I., Mitani-Ueno, N., Ma, J. F., Naito, S., and Takano, J. (2017) Polar Localization of the NIP5;1 Boric Acid Channel is Maintained by Endocytosis and Facilitates Boron Transport in Arabidopsis Roots. Plant Cell 10.1105/tpc.16.00825.






Leave a Reply
Want to join the discussion?Feel free to contribute!