The Role of Trigger Factor in Chloroplasts
Chloroplasts contain a small genome that encodes only a minor fraction of approximately 60 to 100 proteins of the entire 3,000 proteins localized in the chloroplast. Chloroplast-encoded proteins, however, are major subunits of important protein complexes involved in gene expression and photosynthesis. The gene expression and biogenesis of plastid proteins is achieved by many components that are still most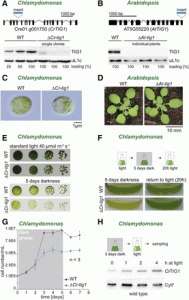 closely related to their cyanobacterial ancestors, including RNA polymerase, the 70S ribosome, and diverse chaperones. One such chaperone is trigger factor (TF), which is known to bind most newly synthesized proteins in bacteria, thereby assisting their correct folding and maturation. In Escherichia coli, TF (EcTF) assist in the biogenesis of all synthesized proteins. The question of how similar molecular chaperones regulate the proteome of chloroplasts remains poorly understood. In this issue, Rohr et al. (10.1104/pp.18.01252) present a functional investigation of chloroplast-localized TF (TIG1) in the green alga Chlamydomonas reinhardtii and the vascular land plant Arabidopsis. The authors have previously reported that TIG1s have a dragon-like shape, similar to the conformation of bacterial TF, albeit with slightly altered domain arrangement and flexibility. This structural conservation, despite low amino acid sequence homology E. coli TF, illustrates a remarkable evolutionary robustness of chaperone conformations across various kingdoms of life. Here, the authors report that chloroplastic TF evolved as a specialized chaperone. Unlike other plastidic chaperones that are functionally interchangeable with their prokaryotic counterpart, TIG1 was not able to complement the broadly acting ortholog in Escherichia coli. Whereas general chaperone properties such as the prevention of aggregates or substrate recognition seems to be conserved between bacterial and plastidic TFs, plant TIG1s differed by associating with only a relatively small population of translating ribosomes. Furthermore, a reduction of plastidic TIG1 levels leads to deregulated protein biogenesis. These results suggest a central role of TIG1 in protein biogenesis in the chloroplast.
closely related to their cyanobacterial ancestors, including RNA polymerase, the 70S ribosome, and diverse chaperones. One such chaperone is trigger factor (TF), which is known to bind most newly synthesized proteins in bacteria, thereby assisting their correct folding and maturation. In Escherichia coli, TF (EcTF) assist in the biogenesis of all synthesized proteins. The question of how similar molecular chaperones regulate the proteome of chloroplasts remains poorly understood. In this issue, Rohr et al. (10.1104/pp.18.01252) present a functional investigation of chloroplast-localized TF (TIG1) in the green alga Chlamydomonas reinhardtii and the vascular land plant Arabidopsis. The authors have previously reported that TIG1s have a dragon-like shape, similar to the conformation of bacterial TF, albeit with slightly altered domain arrangement and flexibility. This structural conservation, despite low amino acid sequence homology E. coli TF, illustrates a remarkable evolutionary robustness of chaperone conformations across various kingdoms of life. Here, the authors report that chloroplastic TF evolved as a specialized chaperone. Unlike other plastidic chaperones that are functionally interchangeable with their prokaryotic counterpart, TIG1 was not able to complement the broadly acting ortholog in Escherichia coli. Whereas general chaperone properties such as the prevention of aggregates or substrate recognition seems to be conserved between bacterial and plastidic TFs, plant TIG1s differed by associating with only a relatively small population of translating ribosomes. Furthermore, a reduction of plastidic TIG1 levels leads to deregulated protein biogenesis. These results suggest a central role of TIG1 in protein biogenesis in the chloroplast.