The importance of being in the right place to respond to light cues
Christian Fankhauser, Centre for Integrative Genomics, Faculty of Biology and Medicine, Génopode Building, University of Lausanne, CH–1015 Lausanne, Switzerland
Background: Plants can orient their leaves towards the light. This is known as phototropism and fascinated scientists since Charles Darwin. Phototropism is initiated by a blue light photoreceptor called phototropin. Light activates phototropin’s protein kinase activity (an enzyme that phosphorylates proteins). This is followed by a series of poorly understood events, which lead to the redistribution of the growth hormone auxin in the stem resulting in growth towards the light. PHYTOCHROME KINASE SUBSTRATE 4 (PKS4) is phosphorylated by phototropin and is believed to act between phototropin light activation and auxin redistribution. The mechanism of PKS4 action is unknown because the protein does not contain any domains of known biochemical activity. PKS4 associates with the plasma-membrane, where it was postulated to act.
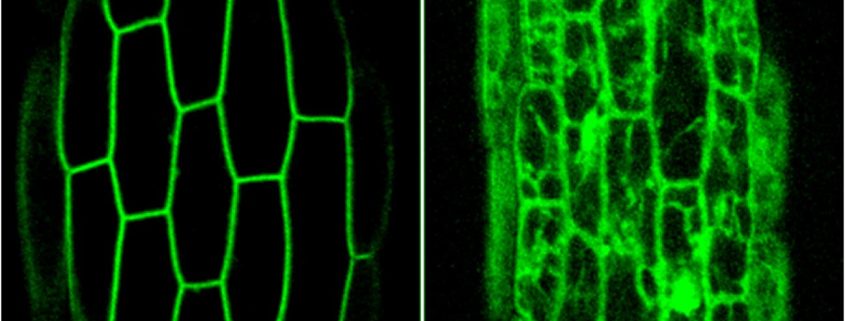
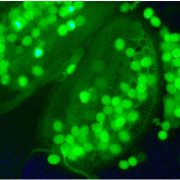
Question: We wanted to understand how PKS4 associates with the plasma membrane and determine whether this subcellular localization is important for its biological activity.
Findings: Functionally important parts of proteins are typically conserved over evolutionary timescales. We therefore identified genes coding for PKS proteins and found that they are present in seed plants and comprise 6 conserved sequence motifs. We showed that one of these motifs is important for efficient PKS association with the plasma membrane in Arabidopsis. Cysteine residues of this motif are modified with lipids, which presumably contribute to plasma membrane association. Moreover, in PKS4 these residues are important for its function in phototropin signaling in Arabidopsis.
Next steps: Our study identified a PKS4 sequence motif that is important for biological activity and subcellular localization. However, we still don’t know what the rest of the protein does and how it may be important in linking phototropin activation and auxin redistribution. We hope that studying other conserved motives identified here will allow us to answer this question.
Reference:
Ana Lopez Vazquez, Laure Allenbach Petrolati, Martina Legris, Christophe Dessimoz, Edwin R. Lampugnani, Natasha Glover, and Christian Fankhauser (2023) Protein S-acylation controls the subcellular localization and biological activity of PHYTOCHROME KINASE SUBSTRATE. https://doi.org/10.1093/plcell/koad096