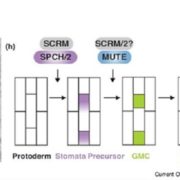
The biogenesis of CLEL peptides involves several processing events in consecutive compartments of the secretory pathway (eLIFE)
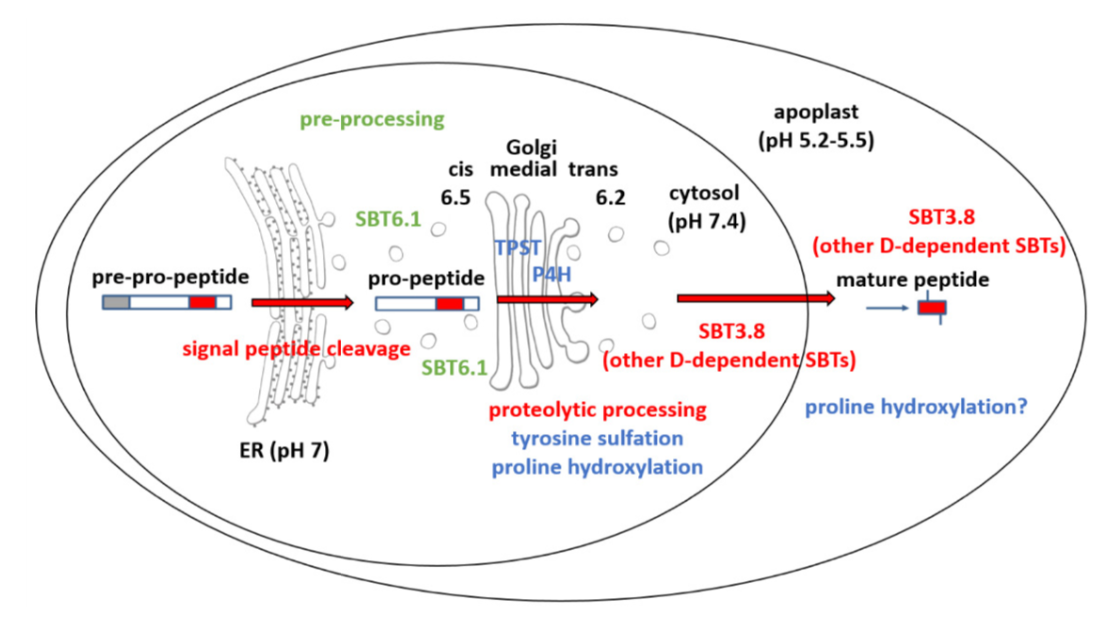 Small signaling peptides are cleaved from precursor proteins by the action of proteases and are also subject to other post-translational modifications. Subtilases (SBT) are mostly extracellular proteases, but SBT6.1 is membrane-localized at the Golgi and plasma membranes. Furthermore, it has been shown to cleave precursors of the CLE-Like (CLEL) peptides, specifically CLEL6 and 9, which are involved in root gravitropism in an SBT6.1-dependent manner. Where and when these proteolytic activities happen in the cell is not known. In this paper, Stührwohldt et al. used cell biology and genetic experiments to show that CLEL6 and 9 are proteolytically processed after exit from endoplasmic reticulum by SBT6.1 and SBT3.8 as they travel through the Golgi, post-Golgi compartment and apoplast. SBT6.1 acts mainly in the Golgi, and cleavage of the pro-CLEL6 to immature CLEL6 is necessary for it to move onwards through the secretory pathway. Post-translational modification is also required for subsequent activity by SBT3.8 mostly in post-Golgi compartment or apoplast resulting in mature peptide. This study demonstrates “the complexity of post-translational precursor maturation allowing for stringent control of peptide biogenesis.” (Summary by Vijaya Batthula @Vijaya_Batthula) eLIFE 10.7554/eLife.55580
Small signaling peptides are cleaved from precursor proteins by the action of proteases and are also subject to other post-translational modifications. Subtilases (SBT) are mostly extracellular proteases, but SBT6.1 is membrane-localized at the Golgi and plasma membranes. Furthermore, it has been shown to cleave precursors of the CLE-Like (CLEL) peptides, specifically CLEL6 and 9, which are involved in root gravitropism in an SBT6.1-dependent manner. Where and when these proteolytic activities happen in the cell is not known. In this paper, Stührwohldt et al. used cell biology and genetic experiments to show that CLEL6 and 9 are proteolytically processed after exit from endoplasmic reticulum by SBT6.1 and SBT3.8 as they travel through the Golgi, post-Golgi compartment and apoplast. SBT6.1 acts mainly in the Golgi, and cleavage of the pro-CLEL6 to immature CLEL6 is necessary for it to move onwards through the secretory pathway. Post-translational modification is also required for subsequent activity by SBT3.8 mostly in post-Golgi compartment or apoplast resulting in mature peptide. This study demonstrates “the complexity of post-translational precursor maturation allowing for stringent control of peptide biogenesis.” (Summary by Vijaya Batthula @Vijaya_Batthula) eLIFE 10.7554/eLife.55580