SUMO reins in an overactive cell cycle
Lin et al. investigate how SUMOylation of a ribosomal protein affects cell size-mediated cell cycle control in Chlamydomonas reinhardtii.
Plant Cell https://doi.org/10.1105/tpc.19.00301
By Yen-Ling Lin1,2,3 and Su-Chiung Fang1,2
1Biotechnology Center in Southern Taiwan, Academia Sinica, Tainan, Taiwan
2Agricultural Biotechnology Research Center, Academia Sinica, Taipei, Taiwan
3Ph.D. Program in Microbial Genomics, National Chung Hsing University and Academia Sinica, Taichung, Taiwan
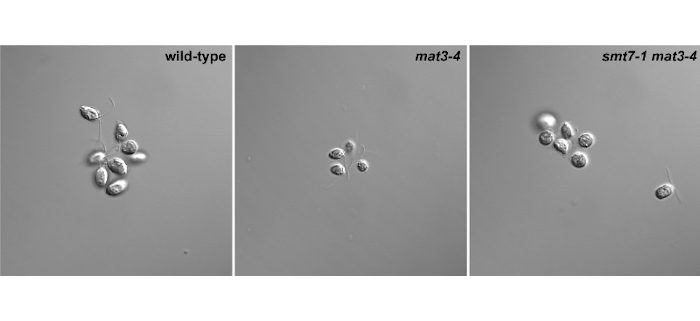
Background: During the cell division cycle, cells regulate their size to achieve cell-size homeostasis. Cell size homeostasis requires coordination of growth and cell division, but the underlying mechanism remains a key question. The regulatory module required for size control is similar in mammals, plants, and the unicellular green microalga Chlamydomonas reinhardtii. Because of its single-cell nature and haploid genome, C. reinhardtii has become a model system to address cell size questions. The retinoblastoma (Rb) tumor suppressor pathway is important to control size checkpoint functions in C. reinhardtii and loss of the Chlamydomonas Rb gene, MAT3, increases cell division number and yields small cells. We previously identified a small ubiquitin modifier (SUMO) protease, SMT7, that acts downstream of the Rb tumor suppressor pathway. smt7-1 mat3-4 cells were larger than mat3 cells, but smaller than wild-type cells.
Question: We wanted to validate the SUMO protease activity of SMT7 and identify the SMT7 targets whose SUMOylation regulation is important for MAT3-dependent size checkpoint functions.
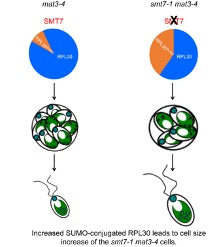 Findings: We demonstrated that SMT7 is a SUMO protease and its activity is required for MAT3-dependent cell size control. We identified ribosomal protein L30 (RPL30) as the key target of SMT7. Lack of SMT7 function in the smt7-1 mat3 strain caused an increase in SUMO-conjugated RPL30 protein, reducing the cell division number and increasing cell size. Together, our study reveals a novel mechanism through which SUMO conjugation regulates RPL30 to control cell division in mat3 mutant cells., terpene oxygenation modifies the composition of the flower microbial population.
Findings: We demonstrated that SMT7 is a SUMO protease and its activity is required for MAT3-dependent cell size control. We identified ribosomal protein L30 (RPL30) as the key target of SMT7. Lack of SMT7 function in the smt7-1 mat3 strain caused an increase in SUMO-conjugated RPL30 protein, reducing the cell division number and increasing cell size. Together, our study reveals a novel mechanism through which SUMO conjugation regulates RPL30 to control cell division in mat3 mutant cells., terpene oxygenation modifies the composition of the flower microbial population.
Next steps: The discovery that SUMO-conjugated RPL30 regulates size-mediated cell cycle control function opens new questions. How does increased SUMO-conjugated RPL30 protein affect cell division? Ribosome is the microfactory for protein biosynthesis and RPL30 is one of the ribosome components. It is possible that SUMO-conjugated RPL30 affects ribosome synthesis and/or pre-ribosomal particle assembly that subsequently affects cell division. It is also possible that SUMO-conjugated RPL30 regulates protein synthesis by regulating translation specificity. Alternatively, SUMOylated RPL30 may have a function other than protein synthesis. Further investigation of these possibilities will be important for understanding the mechanism connecting RPL30, SUMOylation, and size checkpoint functions.
Yen-Ling Lin, Chin-Lin Chung, Ming-Hui Chen, Chun-Han Chen, Su-Chiung Fang. (2020). SUMO Protease SMT7 Modulates Ribosomal Protein L30 and Regulates Cell-size Checkpoint Function. Plant Cell; DOI: https://doi.org/10.1105/tpc.19.00301