Sugar Is Sweeter: Plants Open Their “Mouths” for Glucose, Not Malate, In the Morning
Regulation of stomatal opening and closing in plants in response to environmental cues continues to be well studied (García-León et al., 2019; Li et al., 2020), as it is important for balancing the intake of carbon dioxide for photosynthesis (PS) with the release of water during transpiration. But more fundamental to this is what enables the mouth-resembling stomatal pore to open in the first place, especially in the morning when plants are first exposed to light.
Starch degradation in the guard cells, the two “lips” of the stoma, is one of the hallmarks of stomatal opening in response to blue light (BL) exposure at dawn. To study this phenomenon, Flütsch et al. (2020), like others (e.g., Li et al., 2020) have taken advantage of the amy3 bam1 double mutant, which lacks functional α-AMYLASE3 and β-AMYLASE1 hydrolases and displays reduced stomatal aperture and opening rate. However, Flütsch et al. (2020) additionally combined this powerful genetics tool with physiology and biochemistry to challenge some earlier assumptions about the relationship between guard cell metabolism and ion transport, and ultimately stomatal opening dynamics.
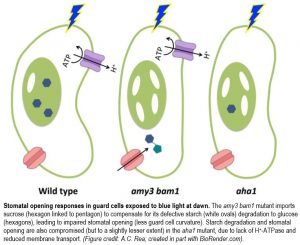 Even prior to the known role of ion transport across the guard cell plasma membrane (PM) in driving the increase in turgor pressure needed for stomatal opening, a long-held view since the 1900s (Lloyd, 1908) was that guard cell starch is broken down into sugars that are converted to malate2− (Mal). However, unexpectedly, the amy3 bam1 mutant, which is defective in guard cell starch degradation, had fully functional PM-localized H+-ATPase, unimpaired ion fluxes, and Mal levels equivalent to those of wild type, even upon exposure to BL at dawn. Glucose (Glc) levels, on the other hand, were maintained between the end of the night (EoN) and BL exposure in wild type, but not in the amy3 bam1 mutant, whose Glc levels decreased in BL. Like Mal, sucrose (Suc) also decreased in BL in both genotypes, but was much more abundant at the EoN in the amy3 bam1 mutant, perhaps because it was transported from the mesophyll as a compensatory mechanism. Thus, it is likely that stomatal opening is driven collectively by (1) Mal and Suc as the energy providers, and (2) Glc as the main metabolite and/or osmolyte produced directly from BL-induced starch degradation.
Even prior to the known role of ion transport across the guard cell plasma membrane (PM) in driving the increase in turgor pressure needed for stomatal opening, a long-held view since the 1900s (Lloyd, 1908) was that guard cell starch is broken down into sugars that are converted to malate2− (Mal). However, unexpectedly, the amy3 bam1 mutant, which is defective in guard cell starch degradation, had fully functional PM-localized H+-ATPase, unimpaired ion fluxes, and Mal levels equivalent to those of wild type, even upon exposure to BL at dawn. Glucose (Glc) levels, on the other hand, were maintained between the end of the night (EoN) and BL exposure in wild type, but not in the amy3 bam1 mutant, whose Glc levels decreased in BL. Like Mal, sucrose (Suc) also decreased in BL in both genotypes, but was much more abundant at the EoN in the amy3 bam1 mutant, perhaps because it was transported from the mesophyll as a compensatory mechanism. Thus, it is likely that stomatal opening is driven collectively by (1) Mal and Suc as the energy providers, and (2) Glc as the main metabolite and/or osmolyte produced directly from BL-induced starch degradation.
Like the amy3 bam1 mutant, the aha1 mutant, which lacks the functional BL-activated PM-localized H+-ATPase in guard cells, displayed reduced starch degradation in response to BL. This alone explains the slower stomatal opening kinetics and reduced stomatal aperture in both mutants because, despite their increased stomatal density and accompanying increase in potential maximum stomatal conductance (gsmax), they had equivalent pore length and depth and reduced gs amplitudes compared to wild type. By contrast, in response to PS-saturating or red-light-only conditions, stomatal opening kinetics were independent of guard cell starch degradation. While the amy3 bam1 mutant had gs responses equivalent to wild type, presumably due to an increase in PS-produced sugar osmolytes transported to the guard cells from the mesophyll, the aha1 mutant had reduced gs responses despite having guard cell starch levels equivalent to that of wild type, suggesting that H+-ATPase is necessary for transport of substances from the mesophyll to effect stomatal opening. Indeed, unlike the aha1 mutant, the wild type exhibited increases in starch and Suc levels in guard cells and efficient stomatal opening, which occurred in intact leaves but not in isolated epidermal peels.
These results are interesting because they suggest that BL-induced stomatal opening is hampered by reduced energy or osmolytes due to impaired starch degradation in the amy3 bam1 mutant, but due to impaired transport of those substances from the mesophyll in the aha1 mutant (see figure). Therefore, coordination of starch metabolism to Glc and osmolyte transport across the PM in guard cells is paramount to proper regulation of this “mouth-opening” mechanism. It seems that humans are not the only ones eager to open their mouths for a bit of sugar in the morning!
Anne C. Rea
Michigan State University
MSU-DOE Plant Research Laboratory
ORCID: 0000-0002-2996-5709
REFERENCES
Flütsch, S., Wang, Y., Takemiya, A., Vialet-Chabrand, S.R.M., Klejchová, M., Nigro, A., Hills, A., Lawson, T., Blatt, M.R., and Santelia, D. (2020). Guard cell starch degradation yields glucose for rapid stomatal opening in Arabidopsis. Plant Cell DOI: https://doi.org/10.1105/tpc.18.00802.
García-León, M., Cuyas, L., El-Moneim, D.A., Rodriguez, L., Belda-Palazón, B., Sanchez-Quant, E., Fernández, Y., Roux, B., Zamarreño, A.M., García-Mina, J.M., Nussaume, L., Rodriguez, P.L., Paz-Ares, J., Leonhardt, N., and Rubio, V. (2019). Arabidopsis ALIX regulates stomatal aperture and turnover of ABA receptors. Plant Cell 31: 2411–2429.
Li, J.-G., Fan, M., Hua, W., Tian, Y., Chen, L.-G., Sun, Y., and Bai, M.-Y. (2020). Brassinosteroid and hydrogen peroxide interdependently induce stomatal opening by promoting guard cell starch degradation. Plant Cell 32: 984–999.
Lloyd, F. (1908). The behaviour of stomata. Carnegie Inst. Washington Publ. 82: 1–42.



