SnRK1 and TOR—in a different light
Saile et al. explore the role of SnRK1 and TOR in light-dependent seedling development and splicing.
https://doi.org/10.1093/plcell/koad168
Jennifer Saile and Andreas Wachter
Institute for Molecular Physiology (imP), University of Mainz
Background: Plants adjust their development to light and metabolic signals to make best use of their available resources. Dark-grown seedlings undergo etiolation with increased hypocotyl elongation, while illumination causes greening of leaves to initiate photosynthesis. These alternative developmental programs require reprograming of many genes via various mechanisms. Accordingly, exposing etiolated seedlings to light or sugar causes changes in splicing, a critical step in the generation of mature mRNAs. Key sensors for metabolic signals are SnRK1 (SNF1-RELATED KINASE 1) and TOR (TARGET OF RAPAMYCIN), which were shown to be activated under conditions of limited and ample energy availability, respectively.
Question: Given the importance of SnRK1 and TOR in sensing the metabolic state, we examined their role in light-dependent seedling development and splicing. We were particularly interested in establishing if they have opposing functions and whether this differs for seedlings grown in the presence or absence of light.
Findings: We have established mutants in the model plant Arabidopsis thaliana for inducible repression of SnRK1 and TOR. Mutant analysis revealed that both components are needed for regular seedling development in darkness and light. Furthermore, SnRK1 and TOR are involved in controlling light-regulated splicing events. Knockdown of either SnRK1 or TOR caused similar changes in splicing as those observed upon exposing etiolated seedlings to light or sucrose. Our findings demonstrate that concurring activities of these two energy sensors are indispensable for proper regulation of gene expression and seedling development.
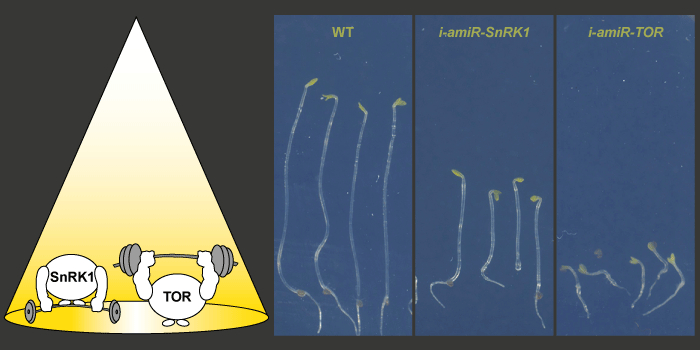
Next steps: SnRK1 and TOR are assumed to have antagonistic functions in energy sensing. Resolving their crosstalk in various developmental conditions will be a key aspect of future research. Moreover, it will be of interest to examine the mechanism by which SnRK1 and TOR can alter the splicing outcome.
Reference:
Jennifer Saile, Theresa Wießner-Kroh, Katarina Erbstein, Dominik M. Obermüller, Anne Pfeiffer, Denis Janocha, Jan Lohmann, and Andreas Wachter. (2023). SNF1-RELATED KINASE 1 and TARGET OF RAPAMYCIN control light-responsive splicing events and developmental characteristics in etiolated Arabidopsis seedlings. https://doi.org/10.1093/plcell/koad168