Tissue-specific Gene Elimination in Plants
Decaestecker and Buono et al. develop a system for tissue-specific gene knockout to enable phenotypic analysis of context-specific gene function.
Plant Cell https://doi.org/10.1105/tpc.19.00454
By Ward Decaestecker, Rafael Andrade Buono, Moritz Nowack and Thomas Jacobs
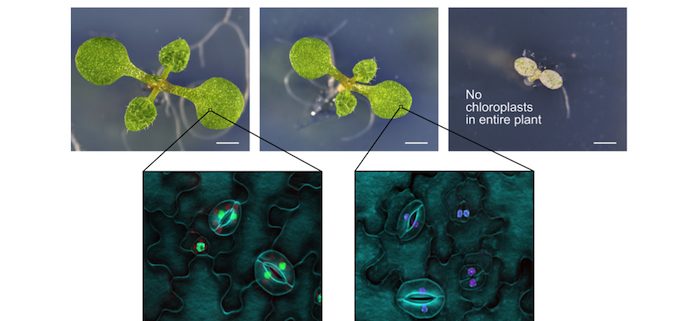
Background: As plant molecular geneticists, we work to understand how gene functions shape plant growth and development. Our standard approach to study gene function has been to first eliminate (or knockout) a gene by mutating the plant’s DNA. These mutations are permanent and can be passed on to the offspring. This allows us to perform experiments comparing plants with and without this individual gene. However, it is thought that ~10% of plant genes are so fundamentally important that it is impossible to make such a mutant without killing or severely affecting the entire plant. This prevents us from studying the functions of some really important genes. For example, PDS3 is an essential gene for chloroplast formation, and conventional mutants are completely white and cannot survive for very long.
Question: We wanted to develop a technology to overcome this restriction. For that we turned to CRISPR technology, as it can very effectively generate DNA mutations. We wanted to see if it was possible to make DNA mutations only in specific cells by having CRISPR activated only in those cell types.
Findings: We developed a CRISPR system we call CRISPR-TSKO for tissue-specific knockout. We demonstrate the use of CRISPR-TSKO by mutating several essential genes in either different parts of the root or stomata (small openings on the leaves facilitating air exchange) in Arabidopsis thaliana. The mutated plants all grew normally, but upon closer inspection of the roots or stomatal cells, we could see the effects of the mutations we made. For example, when we targeted PDS3 specifically in the stomata, plants developed normally but lacked chloroplasts in the stomatal cells. These plants can now be used to investigate the specific role of chloroplasts in stomatal function. In addition, we made a user-friendly toolkit that allows us to easily modify CRISPR-TSKO so it is active in different cells or to easily target different genes.
Next Steps: The versatility of CRISPR-TSKO will enable us and other researchers to mutate and investigate the functions of fundamentally important genes in specific cells, tissues, or organs during various processes of plant growth and development.
Ward Decaestecker, Rafael Andrade Buono, Marie L. Pfeiffer, Nick Vangheluwe, Joris Jourquin, Mansour Karimi, Gert Van Isterdael, Tom Beeckman, Moritz K. Nowack, and Thomas B. Jacobs (2019). CRISPR-TSKO: A Technique for Efficient Mutagenesis in Specific Cell Types, Tissues, or Organs in Arabidopsis. Plant Cell. https://doi.org/10.1105/tpc.19.00454.
Key words: CRISPR, gene editing, Arabidopsis, gene expression, tissue-specific