An Unexpected Role of a Phosphatase-like Protein in Starch Degradation
Schreier et al. uncover the surprising role played by the glucan phosphatase family member, LIKE SEX4 1, as a protein scaffold on the starch granule surface that binds β-amylases. Plant Cell https://doi.org/10.1105/tpc.19.00089.
By Tina B. Schreier1,2 and Samuel C. Zeeman1
1 Institute of Molecular Plant Biology, ETH Zurich, CH-8092 Zurich, Switzerland.
2 Current address: Department of Plant Sciences, University of Cambridge, Downing Street, Cambridge CB2 3EA, UK.
Background: Starch is synthesized in leaf chloroplasts during the day and is degraded at night to sustain metabolism when photosynthesis is not possible. Starch exists as semi-crystalline granules composed of glucose polymers (glucans). The crystalline granule surface presents a challenge for starch-degrading enzymes. The phosphorylation of glucan chains helps reduce crystallinity and increase accessibility for degradative enzymes such as β-amylases (BAMs). However, the complete degradation of these chains requires the phosphates to be removed again. Thus, glucan phosphatases play an important role in starch degradation.
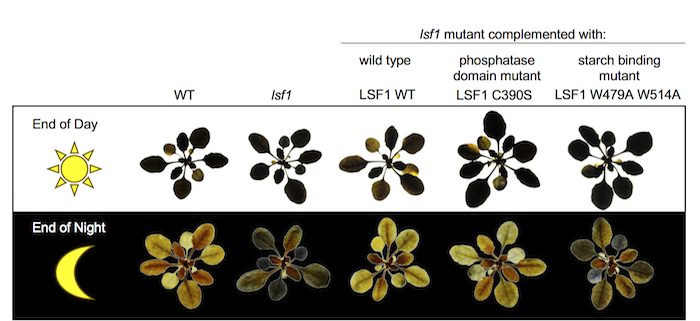
Question: LSF1, a member of the glucan phosphatase family, is required for normal starch degradation in Arabidopsis. The lsf1 mutant fails to degrade all its leaf starch during the night and thus accumulates excess starch. Unlike other glucan phosphatases, the exact role of LSF1 in starch degradation is unknown. We characterized LSF1 both in vitro and in vivo, focusing on the functions of its predicted dual specificity phosphatase (DSP) domain and its carbohydrate-binding module (CBM48).
Findings: No phosphatase activity was detectable for LSF1 protein in vitro, which led us to hypothesize that it plays a role other than dephosphorylating glucans during starch degradation. We expressed a version of LSF1 with a mutated DSP domain in lsf1 plants. The mutated protein restored normal starch degradation in the mutant background, showing that LSF1 protein, but not phosphatase activity, is required for starch degradation. However, expression of an LSF1 containing mutations in the CBM48 domain was not able to complement the lsf1 mutant, indicating that the starch-binding activity of LSF1 is required for normal starch degradation. LSF1 interacts with the two major BAM isoforms in the chloroplast, BAM1 and BAM3. We propose that LSF1 facilitates effective starch degradation by acting as a BAM-binding scaffold at the starch granule surface.
Next steps: Further research is needed to assess the dynamics of BAM-LSF1 complex formation and understand its role in controlling nighttime starch degradation. It is also time to explore the role of LSF1 when plants degrade starch in response to various abiotic stresses such as drought, or in specific cell types such as guard cells.
Tina B. Schreier, Martin Umhang, Sang-Kyu Lee, Wei-Ling Lue, Zhouxin Shen, Dylan Silver, Alexander Graf, Antonia Müller, Simona Eicke, Martha Stadler-Waibel, David Seung, Sylvain Bischof, Steven P. Briggs, Oliver Kötting, Greg B. G. Moorhead, Jychian Chen, and Samuel C. Zeeman. (2019). LIKE SEX4 1 Acts as a β-amylase-binding Scaffold on Starch Granules During Starch Degradation. Plant Cell 31: xxx; DOI: https://doi.org/10.1105/tpc.19.00089
Key words: starch, protein-protein interactions, chloroplast, Arabidopsis