Posttranslational regulation of photosynthetic activity via the TOR kinase in plants
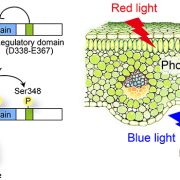
 In the dazzling dance of plant cellular life, chloroplasts must groove in harmony with growth to sidestep the perils of photooxidative damage. This harmony between photosynthesis and photooxidative damage is orchestrated by the guanosine tetraphosphate (ppGpp) signalling pathway which traces its roots back to prokaryotic origins. RelA SpoT homolog 3 (RSH3), a nuclear-encoded enzyme, is crucial for ppGpp biosynthesis, which negatively regulates photosynthesis. The LST8 subunit of the TOR complex, a growth-promoting kinase, interacts with and phosphorylates the N-terminal region (NTR) of RSH3, highlighting a post-translational regulatory mechanism. The localization of RSH3 is intricately linked to its NTR’s phosphorylation status; the phosphomimic version of RSH3 oscillates between chloroplast and nucleocytosolic locations, while the phospho-null version shows chloroplastic localization. Inhibiting TOR disrupts the phosphorylation, leading to increased presence of RSH3 in the chloroplasts, elevated ppGPP levels, and inhibition of photosynthesis. This TOR-centric regulation of ppGPP levels and photosynthesis offers a rapid response mechanism ensuring coordinated growth and photosynthesis, that might be handy especially under stress conditions. Ultimately, this study reveals a sophisticated mechanism where TOR keeps chloroplasts in check, preventing oxidative mayhem and ensuring the plant’s survival. (Summary by Nibedita Priyadarshini) Science Advances. 10.1126/sciadv.adj3268
In the dazzling dance of plant cellular life, chloroplasts must groove in harmony with growth to sidestep the perils of photooxidative damage. This harmony between photosynthesis and photooxidative damage is orchestrated by the guanosine tetraphosphate (ppGpp) signalling pathway which traces its roots back to prokaryotic origins. RelA SpoT homolog 3 (RSH3), a nuclear-encoded enzyme, is crucial for ppGpp biosynthesis, which negatively regulates photosynthesis. The LST8 subunit of the TOR complex, a growth-promoting kinase, interacts with and phosphorylates the N-terminal region (NTR) of RSH3, highlighting a post-translational regulatory mechanism. The localization of RSH3 is intricately linked to its NTR’s phosphorylation status; the phosphomimic version of RSH3 oscillates between chloroplast and nucleocytosolic locations, while the phospho-null version shows chloroplastic localization. Inhibiting TOR disrupts the phosphorylation, leading to increased presence of RSH3 in the chloroplasts, elevated ppGPP levels, and inhibition of photosynthesis. This TOR-centric regulation of ppGPP levels and photosynthesis offers a rapid response mechanism ensuring coordinated growth and photosynthesis, that might be handy especially under stress conditions. Ultimately, this study reveals a sophisticated mechanism where TOR keeps chloroplasts in check, preventing oxidative mayhem and ensuring the plant’s survival. (Summary by Nibedita Priyadarshini) Science Advances. 10.1126/sciadv.adj3268