Pollen tubes use matrix rigidity to direct growth
Emily R Larson
School of Biological Science, University of Bristol, Bristol, UK
ORCID: 0000-0002-5498-8152
Pollen tube growth through plant pistil tissues requires interactions between the male and female cells to regulate compatibility (McCormick, 1998), and hormonal and chemical signals that guide the pollen tube down through the style to the ovaries (reviewed in: Cameron and Geitmann, 2018; Li et al., 2018). Pollen tubes are specialized cells that grow by tip growth, which requires sustained turgor pressure and vesicle trafficking to support expansion at the apex of the pollen tube (reviewed in Grebnev et al., 2017; Lord, 2000). In the current issue, Reimann et al. (2020) provide a useful method for estimating the force pollen tubes generate during tip growth through solid substrates of differing stiffness and describe an active mechanosensory mechanism they term durotropism (with duro referring to hardness) that directs pollen tube growth through stiff transmission environments.
 Depending on flower anatomy, pollen tubes must navigate different transmission tissues of the stigma and style. Some plants, like Arabidopsis thaliana and Nicotiana tabacum have solid styles that require the pollen tube to grow through cell extracellular matrices; while plants like Lilium longiflorum (easter lily) and Eschscholzia californica (California poppy) have hollow styles composed of viscous extracellular matrix or papilla-like cells that the pollen tube can pass through and over, respectively (Becker et al., 2005; Sanders and Lord, 1992). While there is empirical evidence that pollen from plants with different style composition grow better on appropriately stiff or soft agarose gel media (Ghanbari et al., 2018; Gossot and Geitmann, 2007), why this is the case has not been established.
Depending on flower anatomy, pollen tubes must navigate different transmission tissues of the stigma and style. Some plants, like Arabidopsis thaliana and Nicotiana tabacum have solid styles that require the pollen tube to grow through cell extracellular matrices; while plants like Lilium longiflorum (easter lily) and Eschscholzia californica (California poppy) have hollow styles composed of viscous extracellular matrix or papilla-like cells that the pollen tube can pass through and over, respectively (Becker et al., 2005; Sanders and Lord, 1992). While there is empirical evidence that pollen from plants with different style composition grow better on appropriately stiff or soft agarose gel media (Ghanbari et al., 2018; Gossot and Geitmann, 2007), why this is the case has not been established.
Using elegant experiments to test how pollen tubes grow through different concentrations of agarose media, the authors show that pollen tubes from species with solid styles increase their growth rate as medium stiffness increases, while those from species with hollow styles do not. Their results suggest that there is an active mechanosensitive response to environmental hardness that can enhance the growth of pollen tubes from solid-style species, reminiscent of the durotactic movement of mammalian mesenchymal cells towards rigid growth substrates (Lo et al., 2000), but this type of mechanosensing behaviour has not been described in plants.
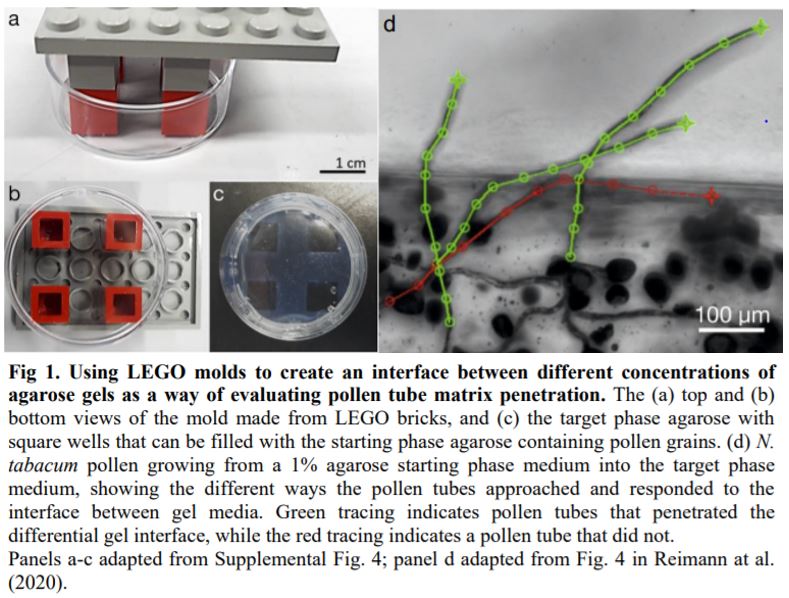
Reimann at al. (2020) initially established a method for calculating penetration forces by pushing a blunt pin with known dimensions through different concentrations of agarose gel at a steady rate. By using the pin as a proxy for a pollen tube, they confirmed that measuring pollen tube tip diameter and growth rate from time-lapse images could estimate pollen tube penetration forces. These forces have been measured before using capacitance force sensors in bespoke microchips (Agudelo et al., 2013; Burri et al., 2018; Ghanbari et al., 2018), but these tools can miss small forces outside the sensor’s range of detection. With clever setups that tested how fast pollen tubes grew from one agarose concentration into a different concentration, the authors showed that changes in pollen tube growth rates depend on medium stiffness and plant species. More interesting still, by measuring the ability of pollen tubes to penetrate an interface between two gel stiffnesses, the authors show that pollen tubes from plants with solid styles prefer a stiffer growth matrix (Fig. 1).
This report establishes that pollen tube growth behaviour is correlated with mechanical differences in transmission tract physiology and adds another layer of regulation to pollen tube growth and plant fertilization mechanics. Quantitative models such as the one presented in this paper improve our ability to measure small but meaningful dynamics that control pollen tube growth and may have application in other tip-growing cell types. This work also provides interesting developments in our understanding of the interplay between cells and their environments.
References
Agudelo, C.G., Sanati Nezhad, A., Ghanbari, M., Naghavi, M., Packirisamy, M., and Geitmann, A. (2013). TipChip: a modular, MEMS-based platform for experimentation and phenotyping of tip-growing cells. Plant J. Cell Mol. Biol. 73, 1057–1068.
Becker, A., Gleissberg, S., and Smyth, D.R. (2005). Floral and Vegetative Morphogenesis in California Poppy (Eschscholzia californica Cham.). Int. J. Plant Sci. 166, 537–555.
Burri, J.T., Vogler, H., Läubli, N.F., Hu, C., Grossniklaus, U., and Nelson, B.J. (2018). Feeling the force: how pollen tubes deal with obstacles. New Phytol. 220, 187–195.
Cameron, C., and Geitmann, A. (2018). Cell mechanics of pollen tube growth. Curr. Opin. Genet. Dev. 51, 11–17.
Ghanbari, M., Packirisamy, M., and Geitmann, A. (2018). Measuring the growth force of invasive plant cells using Flexure integrated Lab-on-a-Chip (FiLoC). TECHNOLOGY 06, 101–109.
Gossot, O., and Geitmann, A. (2007). Pollen tube growth: coping with mechanical obstacles involves the cytoskeleton. Planta 226, 405–416.
Grebnev, G., Ntefidou, M., and Kost, B. (2017). Secretion and Endocytosis in Pollen Tubes: Models of Tip Growth in the Spot Light. Front. Plant Sci. 8, 154.
Li, H.-J., Meng, J.-G., and Yang, W.-C. (2018). Multilayered signaling pathways for pollen tube growth and guidance. Plant Reprod. 31, 31–41.
Lo, C., Wang, H., Dembo, M., and Wang, Y. (2000). Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152.
Lord, E. (2000). Adhesion and cell movement during pollination: cherchez la femme. Trends Plant Sci. 5, 368–373.
McCormick, S. (1998). Self-incompatibility and other pollen-pistil interactions. Curr. Opin. Plant Biol. 1, 18–25.
Reimann, R., Kah, D., Mark, C., Dettmer, J., Reimann, T., Gerum, R., Geitmann, A., Fabry, B., Dietrich, P., and Kost, B. (2020). Durotropic growth of pollen tubes. Plant Physiol. https://doi.org/10.1104/pp.19.01505
Sanders, L.C., and Lord, E.M. (1992). The Extracellular Matrix in Pollen Tube Growth. In Angiosperm Pollen and Ovules, E. Ottaviano, M.S. Gorla, D.L. Mulcahy, and G.B. Mulcahy, eds. (New York, NY: Springer), pp. 238–244.


