Phosphocode-dependent functional dichotomy of a common co-receptor in plant signalling (Nature)
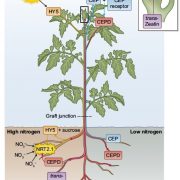
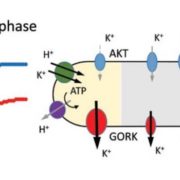
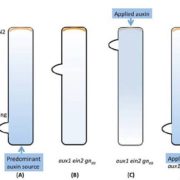
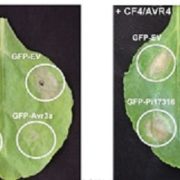
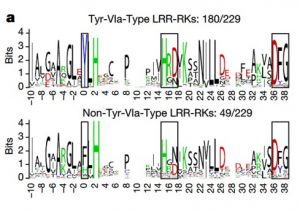 It’s often surprising and a bit confusing when a protein that has been implicated in one process reveals itself to be involved in a totally different process as well. BAK1 (also known as SERK3) was first identified as a co-receptor for brassinosteroids (BRs), forming ligand-induced heterodimers with BRI1, a leucine-rich repeat receptor kinases (LRR-RKs). BAK1 was later found to also form heterodimers with key LRR-RK immune receptors including FLS2 and EFR. Unravelling the specificity of these interactions has been complicated because epitope tagging BAK1 interfered with its activity. Perakki et al. instead tagged the FLS2 and EFR co-receptors and purified the complex, and then identified where the co-purified BAK1 proteins were phosphorylated (they also minimized BR-mediated phosphorylation by pre-treating with a BR-synthesis inhibitor). The authors identified several Ser, Thr and Tyr residues that are phosphorylated when BAK1 associates with FLS2 or EFR; replacing these with non-phosphorylatable Ala blocks immune signaling. One particular Tyr, Y403 in BAK1, is conserved amongst some but not all LRR-RKs and their co-receptors, this revealing a phosphocode that distinguishes two classes of LRR-RKs. (Summary by Mary Williams) Nature 10.1038/s41586-018-0471-x
It’s often surprising and a bit confusing when a protein that has been implicated in one process reveals itself to be involved in a totally different process as well. BAK1 (also known as SERK3) was first identified as a co-receptor for brassinosteroids (BRs), forming ligand-induced heterodimers with BRI1, a leucine-rich repeat receptor kinases (LRR-RKs). BAK1 was later found to also form heterodimers with key LRR-RK immune receptors including FLS2 and EFR. Unravelling the specificity of these interactions has been complicated because epitope tagging BAK1 interfered with its activity. Perakki et al. instead tagged the FLS2 and EFR co-receptors and purified the complex, and then identified where the co-purified BAK1 proteins were phosphorylated (they also minimized BR-mediated phosphorylation by pre-treating with a BR-synthesis inhibitor). The authors identified several Ser, Thr and Tyr residues that are phosphorylated when BAK1 associates with FLS2 or EFR; replacing these with non-phosphorylatable Ala blocks immune signaling. One particular Tyr, Y403 in BAK1, is conserved amongst some but not all LRR-RKs and their co-receptors, this revealing a phosphocode that distinguishes two classes of LRR-RKs. (Summary by Mary Williams) Nature 10.1038/s41586-018-0471-x