Molecular Snapshots of the AKT1-CIPK23 Complex Involved in K+ Uptake
Dhineshkumar Thiruppathi 1,2
Donald Danforth Plant Science Center,
Saint Louis, Missouri 63132
1Lead author
2Author for contact: [email protected]
Potassium (K+) is the most abundant intracellular cation in all living organisms and is an important macronutrient for plant growth and development, contributing up to 10% of plant dry weight. K+ plays critical roles in energy metabolism, transport of minerals and metabolites, protein synthesis, charge neutralization, cellular pH homeostasis, and turgor maintenance (White and Karley, 2010). K+ deficiency is a common abiotic stress that influences K+ homeostasis and highlights the benefits of improving K+ use efficiency (KUE) under changing environments (Qin et al., 2019). Understanding the fundamental mechanisms of K+ uptake and transport is a prerequisite for improving KUE.
K+ uptake is mediated by coupled K+ transporters, voltage-gated channel proteins, and their regulators (Véry and Sentenac, 2002). A common characteristic of several K+ channels in plants is the presence of an ankyrin (ANK) repeat domain in the C-terminal region. AKT1 (Arabidopsis K+ transporter 1) plays a direct role in K+ uptake by Arabidopsis (Arabidopsis thaliana) roots in the high- and low-affinity K+ ranges (Rubio et al., 2008). AKT1 is a shaker-like K+ channel and functions as a tetramer composed of subunits with a C-terminal cytoplasmic segment that includes an ANK repeat domain for channel regulation and an N-terminal transmembrane segment for ion transport. Under low K+ conditions, the ANK domain mediates AKT1 phosphorylation by interacting with and stabilizing a specific protein kinase complex calcineurin B-like protein1/9 (CBL1/9)-CBL-interacting protein kinase23 (CIPK23) that activates K+ influx (Lee et al., 2007). Additional ANK motif-mediated connections between K+ channels and CBLs or CIPKs have been suggested to provide regulatory networks in the plant’s response to a broad range of K+ levels. Additional (Lee et al., 2007; Lefoulon et al., 2016). Understanding the function of the ANK domain of AKT1 and deciphering the CIPK-mediated regulation of AKT1 at the biochemical and structural levels would provide a useful framework for establishing the fundamental role of K+ channels in controlling K+ homeostasis Understanding.
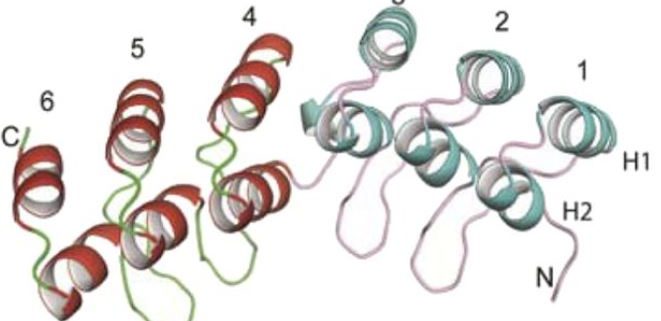
 In this issue, Sanchez-Barrena et al. (2020) shed light on how CIPK23 attaches to the AKT1 channel. The authors focus on the shallow concave side of the ANK domain, which includes the residues specifying the interaction with CIPK23. X-ray crystallography revealed an interruption in the β-sheet structure of this domain at one of the six ANK repeats, leaving the domain distorted and more concave (Fig. 1A). Variable acidic residues between the ANK repeats face toward the concave side, resulting in an asymmetric charge distribution (Fig. 1B). These structural peculiarities have been preserved in the ANK domains of AKT1-like channels of different plant species, with the exception of Arabidopsis AKT2.
In this issue, Sanchez-Barrena et al. (2020) shed light on how CIPK23 attaches to the AKT1 channel. The authors focus on the shallow concave side of the ANK domain, which includes the residues specifying the interaction with CIPK23. X-ray crystallography revealed an interruption in the β-sheet structure of this domain at one of the six ANK repeats, leaving the domain distorted and more concave (Fig. 1A). Variable acidic residues between the ANK repeats face toward the concave side, resulting in an asymmetric charge distribution (Fig. 1B). These structural peculiarities have been preserved in the ANK domains of AKT1-like channels of different plant species, with the exception of Arabidopsis AKT2.
To investigate if the structure of the ANK domain underlies the CIPK23-AKT1 association, the authors conducted a yeast two-hybrid analysis, followed by an electrophysiological analysis of Xenopus oocytes expressing CIPK23-AKT1 modules. Their results indicated that the asymmetric charge distribution on the concave side of the ANK domain is involved in determining the specificity of AKT1 for CIPK23 over other CIPKs. The electrostatic nature of the ANK domain strengthened the CIPK23-AKT1 interaction, which ultimately determined the ability of CIPK23 to activate AKT1 when the CIPK23-AKT1 module was reconstituted in Xenopus oocytes. Thus, the surface properties of the ANK domain of individual K+ channels provide protein-interaction specificity that is involved in their regulation.
The authors next examined whether the ANK domain is essential for AKT1 function and/or stability. They generated an AKT1 protein lacking the entire ANK domain and, additionally, created point mutations that altered the charge distribution of the domain. When these mutant proteins were expressed in a yeast mutant defective in K+ uptake, the yeast grew as well as yeast expressing the full-length AKT1 protein. Sanchez-Barrena et al. concluded that the ANK domain of AKT1 functions only to recruit regulatory proteins, such as CIPK23, to the vicinity of the channel.
Finally, the authors examined the possible coordination of CIPK23 with the ANK domain. Previous efforts to crystalize the ANK domain of AKT1 and the catalytic kinase domain of CIPK23 as a complex had failed (Chaves-Sanjuan et al., 2014; Sanchez-Barrena et al., 2020). Using molecular dynamic simulations informed by biochemical and structural information previously established for these domains (Lee et al., 2007; Chaves-Sanjuán et al., 2014), the authors modeled the CIPK23-ANK complex. This model indicated that the CIPK23 active site and its regulatory activation loop might remain unaltered and solvent accessible upon complex formation with the ANK domain (Fig. 1C), an arrangement that would be consistent with retention of CIPK23 function. Importantly, the polar residues at the concave side of the ANK domain were predicted to be buried within the CIPK23/ANK interface, consistent with their role in dictating the specificity of AKT1 for CIPK23 in biochemical studies.
There is no simple answer to the question of what dictates channel activity. But the devil seems to be in the detail, as small perturbations of the buried surface of the ANK domain of AKT1 specifically alter the binding to CIPK23, enhancing or impairing the ability of CIPK23 to regulate channel activity. The results of this study may provide relevant biotechnological tools for improving the KUE of plants. This study opens the door to addressing a number of questions that have remained unanswered for some time now, such as whether the selective activation of the AKT1 channel depends on the specificity of the CBL1/9-CIPK23 interaction, as suggested by Lee et al. (2007), on the direct interaction of CBL9 with AKT1, as suggested by Grefen and Blatt (2012), on the CIPK23-ANK interaction, or on a combination of these interactions. Sanchez-Barrena et al. do not address this question, but they do offer some tools with which to revisit the problem. Future studies should examine how the CIPK23-ANK interaction arises in planta and establish the effect of this interaction on channel function, as this information is now vital to providing convincing evidence of the relevance of this interaction to AKT1-regulated K+ transport.
LITERATURE CITED
Chaves-Sanjuán A, Sánchez-Barrena MJ, González-Rubio JM, Albert A (2014) Preliminary crystallographic analysis of the ankyrin-repeat domain of Arabidopsis thaliana AKT1: identification of the domain boundaries for protein crystallization. Acta Crystallographica Section F Structural Biology Communications 70: 509–512
Chaves-Sanjuan A, Sanchez-Barrena MJ, Gonzalez-Rubio JM, Moreno M, Ragel P, Jimenez M, Pardo JM, Martinez-Ripoll M, Quintero FJ, Albert A (2014) Structural basis of the regulatory mechanism of the plant CIPK family of protein kinases controlling ion homeostasis and abiotic stress. Proc Natl Acad Sci U S A 111: E4532–41
Grefen C, Blatt MR (2012) Do calcineurin B-like proteins interact independently of the serine threonine kinase CIPK23 with the K+ channel AKT1? Lessons learned from a ménage à trois. Plant Physiol 159: 915–919
Lee SC, Lan W-Z, Kim B-G, Li L, Cheong YH, Pandey GK, Lu G, Buchanan BB, Luan S (2007) A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc Natl Acad Sci U S A 104: 15959–15964
Lefoulon C, Boeglin M, Moreau B, Véry A-A, Szponarski W, Dauzat M, Michard E, Gaillard I, Chérel I (2016) The Arabidopsis AtPP2CA Protein Phosphatase Inhibits the GORK K Efflux Channel and Exerts a Dominant Suppressive Effect on Phosphomimetic-activating Mutations. Journal of Biological Chemistry 291: 6521–6533
Qin Y, Wu W, Wang Y (2019) ZmHAK5 and ZmHAK1 function in K uptake and distribution in maize under low K conditions. Journal of Integrative Plant Biology 61: 691–705
Rubio F, Nieves-Cordones M, Alemán F, Martínez V (2008) Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol Plant 134: 598–608
Véry AA, Sentenac H (2002) Cation channels in the Arabidopsis plasma membrane. Trends Plant Sci 7: 168–175
White PJ, Karley AJ (2010) Potassium Pp: 199-224 In Hell, R. & Mendel, RR (Eds.). Cell Biology of Metals and Nutrient. Plant cell monographs 17: