Mitochondrial dynamics for pollen development
Author: Masanori Izumi
Affiliation: Frontier Research Institute for Interdisciplinary Sciences, Tohoku University, Sendai 980–8578, Japan
Mitochondria are active organelles that move rapidly, change shape and undergo repeated fusion and fission (Frederick and Shaw, 2007; Tilokani et al., 2018). Since mitochondria have many important roles including respiratory energy production through the TCA cycle and oxidative phosphorylation in eukaryotes, the quality and quantity of mitochondria must be controlled in response to environmental conditions, growth stages and cell types.
The mechanism for mitochondrial fusion and fission has been demonstrated in eukaryotes such as yeast, mammals, red algae and land plants (Arimura, 2018). Mitochondrial fission largely requires the function of dynamin-related proteins (DRPs), which have GTPase activity for the scission of vesicles from membrane. In Arabidopsis thaliana plants, DYNAMIN-RELATED PROTEIN 3A (DRP3A) and DRP3B serve to constrict the site for mitochondrial division (Mano et al., 2004; Fujimoto et al., 2009). The leaves of drp3a or drp3b single mutants have elongated, larger mitochondria compared to wild-type plants (Fujimoto et al., 2009). Mitochondrial outer envelope-bound ELONGATED MITOCHONDRIA 1 (ELM1) is a plant-specific protein that is required for the correct localization of DRP3A (Arimura et al., 2008). The elm1 mutant cells also display large, elongated mitochondria. Although mitochondrial fission is impaired in single mutant plants of drp3a, drp3b and elm1, these mutants do not show clear visible abnormal phenotypes during vegetative growth. Therefore, the importance of fission-mediated control of mitochondrial size and shape in plant growth remains unclear.
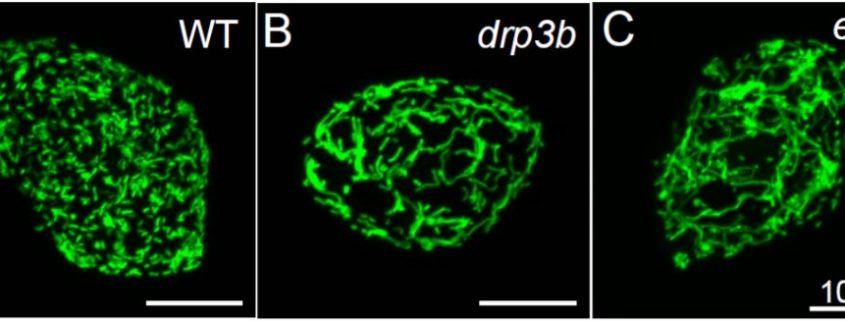
In the current issue of Plant Physiology, Chen et al. (2019) found reduced seed production and impaired pollen maturation, which involves alterations in the production of pollen coat and pollen outer wall, in the drp3a, drp3b and elm1 mutants. Because the formation of the pollen coat and pollen wall is closely related to the function of the tapetum, the authors focused on the changes of mitochondrial behavior in tapetal cells during pollen maturation. Establishment of a 3D-imaging method for mitochondria using fixed, single tapetal cell of transgenic Arabidopsis expressing mitochondrion-targeted GFP driven by a tapetum-specific promoter revealed the dynamic changes in the shape and the number of mitochondria throughout all stages of pollen maturation.
In wild-type plants, during the early stages of pollen maturation, mitochondrial volume per tapetal cell increased, in agreement with the increase in mitochondrial size and cell volume. By contrast, at the later stages, mitochondrial volume drastically decreased although mitochondrial number increased, indicating the accumulation of small, fragmented mitochondria. The changes in mitochondrial behavior in tapetal cells were partly compromised in the Arabidopsis mutants of mitochondrial fission. Total mitochondrial volume per tapetal cell was lower in drp3b and elm1 than in wild-type plants throughout pollen maturation. Additionally, in elm1 plants, tapetal mitochondria were consistently larger and fewer in number relative to those in wild-type plants. These observations suggest that the impaired mitochondrial fission in drp3b and elm1 plants leads to uncontrolled mitochondrial dynamics in tapetal cells, which compromises pollen maturation and seed production.
The ATP content of mature pollen in elm1 plants was comparable to that in wild-type plants, suggesting that the impaired mitochondrial dynamics in the tapetum does not affect the activity of respiratory energy production. Therefore, the precise role of mitochondrial dynamics in pollen maturation is yet to be characterized. The current study established an excellent imaging method to evaluate mitochondrial behavior in tapetal cells, which will facilitate future research elucidating the physiological relevance of mitochondrial dynamics to pollen development.
The current study revealed drastic changes of mitochondrial behavior in tapetal cells during pollen maturation in wild-type plants. Although the increase or decrease in mitochondrial volume requires the synthesis or degradation of mitochondrial components, respectively, how these processes are regulated during pollen development has not been assessed. In the budding yeast Saccharomyces cerevisiae and in Caenorhabditis elegans, the intracellular degradation process autophagy helps to control mitochondrial quantity (Kurihara et al., 2012; Palikaras et al., 2015), however, in plants, our understanding of the contribution of autophagy to mitochondrial degradation is restricted in what occurs in senescing Arabidopsis leaves (Li et al., 2014). How plants control mitochondrial dynamics for pollen development is an interesting question that warrants further study.
FIGURE LEGEND
Figure 1. Tapetal mitochondria in wild-type (A), drp3b (B) and elm1 (C) plants at a binucleate stage during pollen maturation. Single, fixed tapetal cells expressing mitochondrion-targeted GFP controlled by a tapetum-specific promoter were observed via a confocal microscopy. Scale bars = 10 µm. (Adapted from Chen et al., 2019, Fig. 9.)
LITERATURE CITED
Arimura S (2018) Fission and fusion of plant mitochondria, and genome maintenance. Plant Physiol 176: 152-161
Arimura S, Fujimoto M, Doniwa Y, Kadoya N, Nakazono M, Sakamoto W, Tsutsumi N (2008) Arabidopsis ELONGATED MITOCHONDRIA1 is required for localization of DYNAMIN-RELATED PROTEIN3A to mitochondrial fission sites. Plant Cell 20: 1555-1566
Chen PY, Wu CC, Lin CC, Jane WN, Suen DF (2019) 3D imaging of tapetal mitochondria suggests the importance of mitochondrial fission in pollen growth. Plant Physiol
Frederick RL, Shaw JM (2007) Moving mitochondria: Establishing distribution of an essential organelle. Traffic 8: 1668-1675
Fujimoto M, Arimura S, Mano S, Kondo M, Saito C, Ueda T, Nakazono M, Nakano A, Nishimura M, Tsutsumi N (2009) Arabidopsis dynamin-related proteins DRP3A and DRP3B are functionally redundant in mitochondrial fission, but have distinct roles in peroxisomal fission. Plant J 58: 388-400
Kurihara Y, Kanki T, Aoki Y, Hirota Y, Saigusa T, Uchiumi T, Kang D (2012) Mitophagy plays an essential role in reducing mitochondrial production of reactive oxygen species and mutation of mitochondrial DNA by maintaining mitochondrial quantity and quality in yeast. J Biol Chem 287: 3265-3272
Li F, Chung T, Vierstra RD (2014) AUTOPHAGY-RELATED11 plays a critical role in general autophagy- and senescence-induced mitophagy in Arabidopsis. Plant Cell 26: 788-807
Mano S, Nakamori C, Kondo M, Hayashi M, Nishimura M (2004) An Arabidopsis dynamin-related protein, DRP3A, controls both peroxisomal and mitochondrial division. Plant J 38: 487-498
Palikaras K, Lionaki E, Tavernarakis N (2015) Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521: 525-528
Tilokani L, Nagashima S, Paupe V, Prudent J (2018) Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem 62: 341-360