Membrane tethering proteins cooperate in lipid droplet formation
Greer et al. explore the role of SEIPIN in packaging oils into lipid droplets.
Plant Cell https://doi.org/10.1105/tpc.19.00771
By Yingqi Cai, Brookhaven National Laboratory, Department of Biology, Upton, NY 11973
Kent D. Chapman, University of North Texas, BioDiscovery Institute, Denton, TX 76203 USA
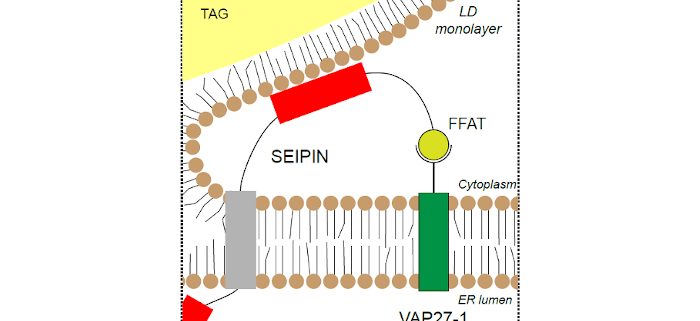
Background: All organisms store energy as fats. In plants this process is mostly associated with seeds and some fruits, which collectively represent the world’s major sources of vegetable oils. However, the cellular processes by which these oils are packaged into lipid droplets (LDs) remain unclear. One protein that appears to be central to this process in plants and other organisms is SEIPIN, originally discovered as a causative gene in some human lipid storage diseases. Plant genomes encode multiple SEIPIN isoforms, and previous research suggested they each make different contributions to LD formation.
Question: Here, we ask if SEIPIN isoforms in plants interact with other protein partners to support LD formation in cells.
Findings: We found that the previously described membrane tethering protein, VAP27-1 (member of the vesicle associated membrane protein- associated protein, VAP, family), interacts with the N-terminus of two of the three Arabidopsis SEIPIN proteins. The extreme ~25 amino acids at the N-terminus of these two SEIPINs contain loosely- conserved phenylalanine-acidic tract (FFAT) motifs that are known binding sites for VAP proteins. With a combination of yeast two-hybrid assays and confocal microscopy studies in planta, evidence indicates that these N-terminal sequences are both necessary and sufficient for the interaction between SEIPINs and VAP27-1. Loss-of-function Arabidopsis mutants of vap27-1 had aberrant, large-sized LDs in their seeds, similar to double knockouts of seipin (sei2 sei3), suggesting that these protein partners may be required for the proper formation of LDs in plants. Taken together, our results point to a previously unrecognized function for VAP27-1 in LD formation.
Next steps: Future studies are aimed at understanding how VAP proteins cooperate with SEIPINs to support the formation of LDs. For example, do they function as tethers to “hold” the LDs in place while they fill with storage lipids? Or, do VAPs actively participate in lipid transfer to the LD during biogenesis? Or, is the interaction with VAPs to mediate release of LDs after filling? Answers to these questions will help provide insights into the cellular production of vegetable oils, a major and growing source of dietary calories for human nutrition.
Michael Scott Greer, Yingqi Cai, Satinder K. Gidda, Nicolas Esnay, Franziska K. Kretzschmar, Damien Seay, Elizabeth McClinchie, Till Ischebeck, Robert T. Mullen, John M. Dyer, Kent D. Chapman. (2020). SEIPIN Isoforms Interact with the Membrane-tethering Protein VAP27-1 for Lipid Droplet Formation. Plant Cell; DOI: https://doi.org/10.1105/tpc.19.00771