Mediator Skills: MED16 Controls Endoreduplication
The discovery of Mediator began with the observation that two transcriptional activators could interfere with each other’s function in vitro, even though they did not bind to the same promoter (Kelleher, Flanagan and Kornberg, 1990). The hypothesis, since then well-validated, was that each transcription factor titrates components (i.e., Mediator) critical for the proper initiation of transcription away from the other transcription factor. The multi-subunit protein complex, Mediator, is mobilized to promoters by transcription factors and in turn can recruit RNA polymerase II.
Core Mediator subunits are generally essential, but components mapping to the Mediator tail are not, and these interact with transcription factors to provide target gene specificity. Arabidopsis thaliana med tail mutants are associated with delayed flowering time and larger organs (med25), decreased tolerance to cold, disease resistance, and iron deficiency (med16), and reduced cell size and higher ploidy levels (med14) compared to the wild type.
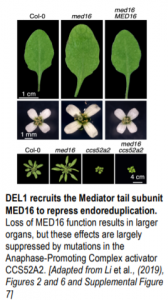
In their article, Liu and colleagues (Liu et al., 2019) describe a new function for MED16 in the control of cell size and DNA ploidy levels and identify the transcription factor and associated target genes responsible. During a forward genetic screen looking for mutants with enlarged leaves and flowers, the authors isolated a new allele of med16. Leaves, petals, and sepals from the mutant are larger, with larger cells, bigger nuclei, and increased DNA ploidy levels compared to the wild type; these phenotypes are indicative of multiple rounds of DNA replication without cell division, i.e., endoreduplication (see Figure). These results demonstrate a role for MED16 in modulating endoreduplication and cell growth.
The authors then turned to a yeast two-hybrid screen and identified DEL1 (DP-E2F-LIKE 1/E2Fe) as the transcription factor that recruits MED16 to chromatin, which solidified the link between MED16 and endoreduplication. Indeed, DEL1 targets are known: the CELL CYCLE SWITCH 52 genes CCS52A1 and CCS52A2, encoding activators of the Anaphase Promoting Complex/Cyclosome (APC/C), an E3 ubiquitin ligase complex that degrades cell cycle proteins. This offered a testable model: DEL1 would bind to the CCS52A1 and CCS52A2 promoters and draw Mediator (via its MED16 subunit) to chromatin and establish transcriptional repression. Left unchecked, CCS52A expression would promote APC/C activity and result in additional rounds of DNA replication.
 Using chromatin immunoprecipitation assays, the authors confirmed that DEL1 and MED16 associate with cis-elements within the CCS52A1 and CCS52A2 promoters. In the case of MED16, this association is dependent on the presence of DEL1, which is consistent with the notion that DEL1 acts as a bridge between MED16 and DNA. Indeed, the expression levels of CCS52A2 in leaves were low in Col-0 and higher in del1 and med16 single and double mutants. The expression of CCS52A1 was also clearly increased in med16 and med16 del1.
Using chromatin immunoprecipitation assays, the authors confirmed that DEL1 and MED16 associate with cis-elements within the CCS52A1 and CCS52A2 promoters. In the case of MED16, this association is dependent on the presence of DEL1, which is consistent with the notion that DEL1 acts as a bridge between MED16 and DNA. Indeed, the expression levels of CCS52A2 in leaves were low in Col-0 and higher in del1 and med16 single and double mutants. The expression of CCS52A1 was also clearly increased in med16 and med16 del1.
Genetic analyses corroborated these results: med16 and ccs52a2 single mutants had opposite phenotypes in terms of cell size and DNA ploidy levels, but the ccs52a1 mutant almost completely suppressed the effects of med16, placing CCS52A1 downstream of MED16 and in the same pathway (see Figure). Similarly, the ccs52a1 mutant partially suppressed the higher ploidy levels of med16, also placing CCS52A1 downstream of MED16. Unfortunately, seedling lethality in ccs52a1 ccs52a2 double mutants prevents the dissection of redundancy between CCS52A genes (Baloban et al., 2013).
MED16 is therefore clearly important for the repression of CCS52A genes by DEL1, but how this gene repression is exerted remains uncertain: Is RNA polymerase II recruited to DEL1 targets by Mediator/MED16, or does Mediator bound to CCS52A promoters include its (inactivating) kinase module?
REFERENCES
Baloban M, Vanstraelen M, Tarayre S, Reuzeau C, Cultrone A, Mergaert P and Kondorosi E. (2013). Complementary and dose-dependent action of AtCCS52A isoforms in endoreduplication and plant size control. New Phytol. 198: 1049-1059.
Kelleher III RJ, Flanagan PM, and Kornberg RD (1990). A Novel Mediator between Activator Proteins and the RNA Polymerase II Transcription Apparatus. Cell 61: 1209-1215.
Liu Z, Chen G, Gao F, Xu R, Li N, Zhang Y, and Li Y (2019). Transcriptional repression of the APC/C activators CCS52A1/A2 by the Mediator complex subunit MED16 controls endoreduplication and cell growth in Arabidopsis. The Plant Cell. Published June 2019. DOI: https://doi.org/10.1105/tpc.18.00811