Maintaining membrane integrity in the face of abiotic stress
Miguel Ángel Botella Mesa – IHSM-UMA-CSIC, Málaga, Spain
Jessica Pérez Sancho – LBM-UMR 5200-CNRS-U.Bordeaux
Noemí Ruiz López – IHSM-UMA-CSIC, Málaga, Spain.
Background: Abiotic stresses such as extreme temperatures and low water availability cause negative effects on plant growth and agricultural productivity. Unlike animals, plants cannot escape from these adverse conditions, therefore they have evolved multiple mechanisms to sense and adapt to these stresses. Among these, lipid signaling occurring at the plasma membrane (PM) after stress sensing controls important plant adaptation processes. One of these PM lipid signals produced at the beginning of the signaling process is diacylglycerol (DAG). However, the accumulation of this lipid in cellular membranes can be toxic, causing the loss of membrane integrity. Therefore, efficient removal of DAG is essential to reset the system and to maintain PM integrity.
Question: We wanted to investigate the mechanisms by which plants control DAG homeostasis at the PM after an episode of abiotic stress.
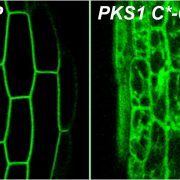
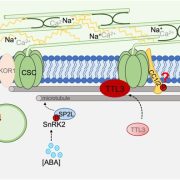
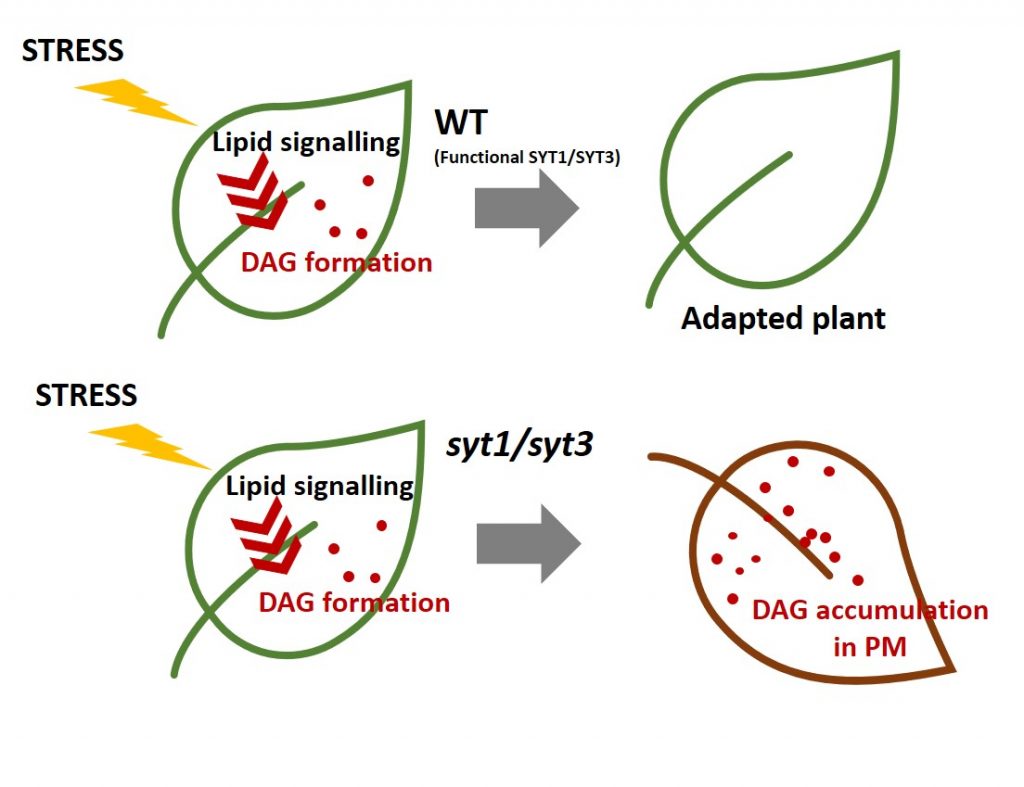 Findings: In this study we analyzed a family of proteins known as synaptotagmins (SYTs), which are essential for plants to tolerate abiotic stress. SYTs are localized in domains where the endoplasmic reticulum (ER) and the PM are very close but not in physical contact; these domains are known as ER-PM contact sites (ER-PM CS). SYT proteins contain a lipid transfer domain supporting a role in lipid transfer between the ER and the PM. We first discovered that in addition to SYT1, its homolog SYT3 is also important to maintain PM integrity under abiotic stress. Cell biology and biochemistry studies determined that these proteins are anchored to the ER and localized at ER-PM CS, where they accumulate during abiotic stress. We then showed that double syt1syt3 mutant plants have higher amounts of DAG after stress treatment at the PM than wild-type plants, suggesting that SYT proteins might transfer DAG from the PM to the ER during abiotic stress. Finally, purification and analysis of lipids bound to SYT1-GFP from leaves supported a role of these proteins in the removal of stress-induced DAG at the PM.
Findings: In this study we analyzed a family of proteins known as synaptotagmins (SYTs), which are essential for plants to tolerate abiotic stress. SYTs are localized in domains where the endoplasmic reticulum (ER) and the PM are very close but not in physical contact; these domains are known as ER-PM contact sites (ER-PM CS). SYT proteins contain a lipid transfer domain supporting a role in lipid transfer between the ER and the PM. We first discovered that in addition to SYT1, its homolog SYT3 is also important to maintain PM integrity under abiotic stress. Cell biology and biochemistry studies determined that these proteins are anchored to the ER and localized at ER-PM CS, where they accumulate during abiotic stress. We then showed that double syt1syt3 mutant plants have higher amounts of DAG after stress treatment at the PM than wild-type plants, suggesting that SYT proteins might transfer DAG from the PM to the ER during abiotic stress. Finally, purification and analysis of lipids bound to SYT1-GFP from leaves supported a role of these proteins in the removal of stress-induced DAG at the PM.
Next steps: Our biochemical data support that SYT1 binds DAG with high specificity. However, studies on extended synaptotagmins (E-Syts), the human SYT orthologs, indicate that E-Syts can bind and transport different classes of glycerolipids. We are interested in understanding the reasons for the specificity of SYT1 and the identification of the mechanism by which plant SYTs transport DAG from the PM to the ER, a process that essential to tolerate abiotic stress.
Noemi Ruiz-Lopez, Jessica Pérez-Sancho, Alicia Esteban del Valle, Richard P Haslam, Steffen Vanneste, Rafael Catalá, Carlos Perea-Resa, Daniël Van Damme, Selene García-Hernández, Armando Albert, José Vallarino, Jinxing Lin, Jiří Friml, Alberto P Macho, Julio Salinas, Abel Rosado, Johnathan A Napier, Vitor Amorim-Silva, Miguel A Botella (2021) Synaptotagmins at the endoplasmic reticulum–plasma membrane contact sites maintain diacylglycerol homeostasis during abiotic stress. Plant Cell. https://doi.org/10.1093/plcell/koab122