Long-distance turgor pressure changes induce local activation of plant glutamate receptor-like channels
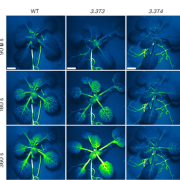
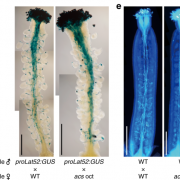
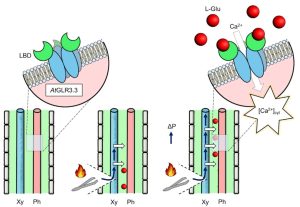 Following wounding or herbivory, plants can rapidly transmit signals systemically (over long distances). These systemic signals are thought to enhance plant defenses by preparing distant tissues for imminent attack. Genetic studies have shown that the glutamate receptor-like calcium channel GLR3.3 (a calcium-permeable channel activated by glutamate binding) is important for systemic signaling; opening this channel allows calcium to flow into cells, raising cytosolic calcium levels ([Ca2+]cyt) and initiating downstream signaling. Here, Grenzi et al. further explored how GLR3.3 acts in systemic signaling, first by demonstrating that the channel is in fact gated through the amino-acid binding site. Previous studies showed an increase in apoplastic L-Glu ([L-Glu]apo) occurs after wounding, which might cause the calcium channels to open. To test this, the authors developed an implantable bioelectronic device that allowed them to modulate [L-Glu]apo in the absence of liquid flow or wounding. They examined its effects in plants expressing fluorescent reporters for both [Ca2+]cyt and [L-Glu]apo. They found that a localized increase in L-Glu did not promote systemic propagating [Ca2+]cyt waves, whereas wounding does and is dependent on GLR3.3. However, systemic increases in [L-Glu]apo are independent of GLR3.3, suggesting that GLR3.3 acts downstream of [L-Glu]apo. How does wounding cause an increase in [L-Glu]apo? Interestingly, turgor pressure induced by wounding is sufficient to raise [L-Glu]apo. A model based on these findings suggests that wound-induced turgor pressure changes act as systemic signals and elevate [L-Glu]apo, which triggers localized [Ca2+]cyt changes via GLR3.3 in tissues removed from the wound site. (Summary by Mary Williams @PlantTeaching) Curr. Biol. 10.1016/j.cub.2023.01.042
Following wounding or herbivory, plants can rapidly transmit signals systemically (over long distances). These systemic signals are thought to enhance plant defenses by preparing distant tissues for imminent attack. Genetic studies have shown that the glutamate receptor-like calcium channel GLR3.3 (a calcium-permeable channel activated by glutamate binding) is important for systemic signaling; opening this channel allows calcium to flow into cells, raising cytosolic calcium levels ([Ca2+]cyt) and initiating downstream signaling. Here, Grenzi et al. further explored how GLR3.3 acts in systemic signaling, first by demonstrating that the channel is in fact gated through the amino-acid binding site. Previous studies showed an increase in apoplastic L-Glu ([L-Glu]apo) occurs after wounding, which might cause the calcium channels to open. To test this, the authors developed an implantable bioelectronic device that allowed them to modulate [L-Glu]apo in the absence of liquid flow or wounding. They examined its effects in plants expressing fluorescent reporters for both [Ca2+]cyt and [L-Glu]apo. They found that a localized increase in L-Glu did not promote systemic propagating [Ca2+]cyt waves, whereas wounding does and is dependent on GLR3.3. However, systemic increases in [L-Glu]apo are independent of GLR3.3, suggesting that GLR3.3 acts downstream of [L-Glu]apo. How does wounding cause an increase in [L-Glu]apo? Interestingly, turgor pressure induced by wounding is sufficient to raise [L-Glu]apo. A model based on these findings suggests that wound-induced turgor pressure changes act as systemic signals and elevate [L-Glu]apo, which triggers localized [Ca2+]cyt changes via GLR3.3 in tissues removed from the wound site. (Summary by Mary Williams @PlantTeaching) Curr. Biol. 10.1016/j.cub.2023.01.042