Keep cool and open up: temperature-induced stomatal opening
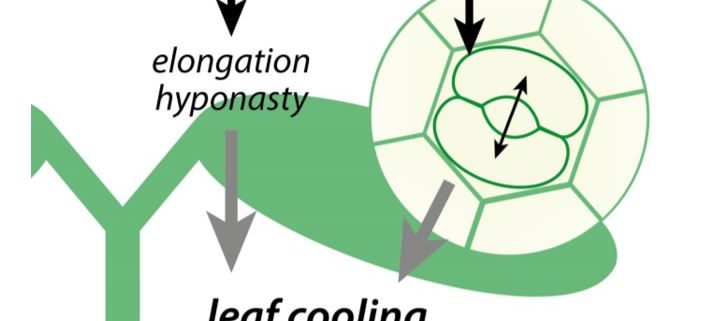
When air temperatures rise above the comfortable level, plants initiate a cooling mechanism to prevent heat stress. High ambient temperatures induce a specific set of growth responses, termed thermomorphogenesis, which includes enhanced hypocotyl and petiole elongation, and leaf hyponasty (reviewed by Casal and Balasubramanian, 2019). These phenotypes are mainly induced via the warm temperature-mediated inactivation of the photoreceptor phytochrome B (phyB) (Jung et al., 2016; Legris et al., 2016). This inactivation stabilizes transcription factor PHYTOCHROME INTERACTING FACTOR4 (PIF4), which promotes cell elongation and thermomorphogenesis (Quint et al., 2016; Casal and Balasubramanian, 2019) (Figure). This ‘open’ architecture allows leaf cooling by the wind (Crawford et al., 2012).
Stomata can additionally help leaf cooling by transpiration. In this issue of Plant Physiology, Kostaki et al. (2020) establish that stomatal aperture is quickly enhanced upon exposure to high temperatures, resulting in a significant reduction in leaf temperature. They demonstrate that this is an epidermis-autonomous response that does not act via the known temperature signaling pathways. Instead, stomatal opening promoted by warm temperatures acts via H+-ATPases and the blue light receptors phototropin1 (phot1) and phototropin2 (phot2).
In Marchantia polymorpha, phototropin acts as a thermosensor that remains in the photo-activated state in cold temperatures, regulating the chloroplast-avoidance response that prevents light damage to the photosystems (Fujii et al., 2017). If this same sensory mechanism exists for Arabidopsis thaliana phot1 and phot2, high temperatures might be expected to promote inactivation. This does, however, not match the loss of warmth-induced stomatal opening in phot1 phot2 loss-of-function plants. As noted by Kostaki et al., the thermosensitive function of the phototropins requires the activation by blue light. However, the stomatal response to high temperatures occurs in darkness as well, confirming the contributions of additional thermosensing mechanisms in the guard cells.
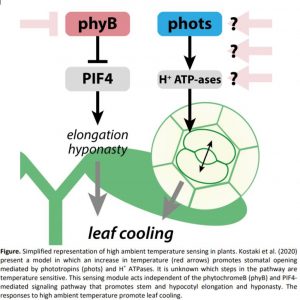 Kostaki and colleagues do not confirm which steps of blue light-mediated stomatal opening are temperature sensitive, but their data support the existence of a sensing mechanism alongside the phyB cascade. The detailed mechanisms remain speculative, particularly around how they are involved in stomatal opening (Figure). In theory, temperature can be sensed by other photoreceptors that are similarly de-activated as is the case for phyB and the phototropins, or by changes in membrane fluidity or RNA translation (discussed by Hayes, 2020). Interestingly, the phyB-PIF4 temperature signaling cascade seems to induce irreversible growth and developmental changes, such as stem elongation, early flowering and stomata development. These traits all help a plant to adapt to a warm environment as the plant develops. By contrast, the stomatal opening phenotype described by Kostaki et al. is rapid and plastic, and probably the earliest response to elevated temperature. This rapid response might therefore require a reversible sensing mechanism that can be switched off when temperatures decrease again. Could different sensing pathways be important in different stages of temperature acclimation and adaptation? Does the location of the sensor system matter? The stomatal phenotype described by Kostaki et al. is clearly localized and guard cell-autonomous, occurring even in isolated epidermal tissue. Does that mean that it does not receive any input from the underlaying tissues? If it does, then how are these inputs communicated between the guard cells and underlying tissues?
Kostaki and colleagues do not confirm which steps of blue light-mediated stomatal opening are temperature sensitive, but their data support the existence of a sensing mechanism alongside the phyB cascade. The detailed mechanisms remain speculative, particularly around how they are involved in stomatal opening (Figure). In theory, temperature can be sensed by other photoreceptors that are similarly de-activated as is the case for phyB and the phototropins, or by changes in membrane fluidity or RNA translation (discussed by Hayes, 2020). Interestingly, the phyB-PIF4 temperature signaling cascade seems to induce irreversible growth and developmental changes, such as stem elongation, early flowering and stomata development. These traits all help a plant to adapt to a warm environment as the plant develops. By contrast, the stomatal opening phenotype described by Kostaki et al. is rapid and plastic, and probably the earliest response to elevated temperature. This rapid response might therefore require a reversible sensing mechanism that can be switched off when temperatures decrease again. Could different sensing pathways be important in different stages of temperature acclimation and adaptation? Does the location of the sensor system matter? The stomatal phenotype described by Kostaki et al. is clearly localized and guard cell-autonomous, occurring even in isolated epidermal tissue. Does that mean that it does not receive any input from the underlaying tissues? If it does, then how are these inputs communicated between the guard cells and underlying tissues?
This work adds to our increasing understanding of high ambient temperature responses in Arabidopsis. Many questions about the sensory mechanism remain unanswered, nonetheless. Importantly, if we want to translate these findings to a natural environment, we will need to ask how the different temperature-induced pathways interact with simultaneous (stress) signals, such as drought or high relative humidity. And if light receptors play such an important role in high temperature acclimation, would it be possible to make plants tolerant to higher temperatures by tweaking the light environment? With global temperatures increasing at an alarming speed, the understanding of these sensing mechanisms will undoubtedly draw increasing scientific interest, and this is likely to provide important breeding targets for future-proof crops.
Charlotte Gommers
Literature Cited
Casal JJ, Balasubramanian S (2019) Thermomorphogenesis. Annual Review of Plant Biology 70: 321-346
Crawford AJ, McLachlan DH, Hetherington AM, Franklin KA (2012) High temperature exposure increases plant cooling capacity. Current Biology 22: R396-R397
Fujii Y, Tanaka H, Konno N, Ogasawara Y, Hamashima N, Tamura S, Hasegawa S, Hayasaki Y, Okajima K, Kodama Y (2017) Phototropin perceives temperature based on the lifetime of its photoactivated state. Proceedings of the National Academy of Sciences 114: 9206-9211
Hayes S (2020) Interaction of Light and Temperature Signalling in Plants. eLS: 1-8
Jung J-H, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Box MS, Charoensawan V, Cortijo S, Locke JC, Schäfer E, Jaeger KE, Wigge PA (2016) Phytochromes function as thermosensors in Arabidopsis. Science 354: 886-889
Kostaki K-I, Coupel-Ledru A, Bonnell VC, Gustavsson M, Sun P, Mclaughlin FJ, Fraser DP, McLachlan DH, Hetherington AM, Dodd AN, Franklin KA (2020) Guard cells integrate light and temperature signals to control stomatal aperture. Plant Physiology: pp.01528.02019 – https://doi.org/10.1104/pp.19.01528
Legris M, Klose C, Burgie ES, Rojas CCR, Neme M, Hiltbrunner A, Wigge PA, Schäfer E, Vierstra RD, Casal JJ (2016) Phytochrome B integrates light and temperature signals in Arabidopsis. Science 354: 897 – 900
Quint M, Delker C, Franklin KA, Wigge PA, Halliday KJ, van Zanten M (2016) Molecular and genetic control of plant thermomorphogenesis. Nature plants 2