Jurassic ZAR; Insights from an atypically conserved immune receptor
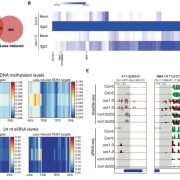
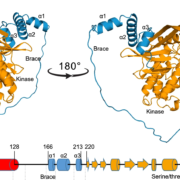
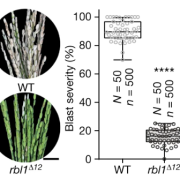
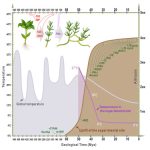
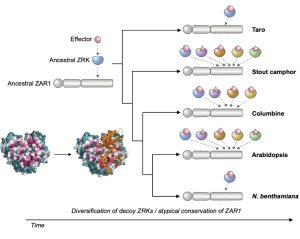 It’s a well-established fact that some proteins are highly variable across species, and others such as histones hardly vary at all. Highly conserved proteins such as histones have very little wiggle room in their structure; small changes can lead to a loss of function. However, for some other proteins variation in sequence and structure is evolutionarily advantageous; proteins that detect pathogens, such as antibodies in humans, are highly variable in order to recognize the huge range of pathogen signatures that might be encountered. Similarly, in plants, there is a high amount of sequence variation in immune receptor proteins, with one notable exception being ZAR1. In this new study, Adachi et al. reconstructed the evolutionary history of this atypically conserved protein. Previously, cryo-EM structural studies of ZAR1 in association with its partner, receptor-like cytoplasmic kinase (RLCK), revealed a resistome complex and key functional domains. In this work, the authors looked at 120 ZAR1 orthologue sequences from 88 species, revealing its highly conserved and more recent features. The authors conclude that, atypically, this protein has changed little since its origins during the Jurassic period. (Interesting aside: the article states the work was initiated during the COVID-19 lockdown, and thanks the Prime Minister of the UK for announcing a stay-at-home order in March 2020; perhaps the first time a Prime Minister has been acknowledged in The Plant Cell?) (Summary by Mary Williams @PlantTeaching) Plant Cell 10.1093/plcell/koad175
It’s a well-established fact that some proteins are highly variable across species, and others such as histones hardly vary at all. Highly conserved proteins such as histones have very little wiggle room in their structure; small changes can lead to a loss of function. However, for some other proteins variation in sequence and structure is evolutionarily advantageous; proteins that detect pathogens, such as antibodies in humans, are highly variable in order to recognize the huge range of pathogen signatures that might be encountered. Similarly, in plants, there is a high amount of sequence variation in immune receptor proteins, with one notable exception being ZAR1. In this new study, Adachi et al. reconstructed the evolutionary history of this atypically conserved protein. Previously, cryo-EM structural studies of ZAR1 in association with its partner, receptor-like cytoplasmic kinase (RLCK), revealed a resistome complex and key functional domains. In this work, the authors looked at 120 ZAR1 orthologue sequences from 88 species, revealing its highly conserved and more recent features. The authors conclude that, atypically, this protein has changed little since its origins during the Jurassic period. (Interesting aside: the article states the work was initiated during the COVID-19 lockdown, and thanks the Prime Minister of the UK for announcing a stay-at-home order in March 2020; perhaps the first time a Prime Minister has been acknowledged in The Plant Cell?) (Summary by Mary Williams @PlantTeaching) Plant Cell 10.1093/plcell/koad175